Home » HSE launches Ireland’s first genetics and genomics strategy
Ireland’s first genetics and geonomics strategy was published in late 2022, following nearly a year of consultation and development. In this article, Dr Mark Bale, Chair of the National Genetics and Genomics Steering Group, provides an overview of the Strategy, while Prof. Eileen Treacy, Clinical Lead at the Irish National Rare Diseases Office, and Prof. Karen A Cadoo, Consultant Cancer Geneticist and Medical Oncologist, offer their views on the importance of genetics and geonomics in providing the best treatment options to Irish patients.

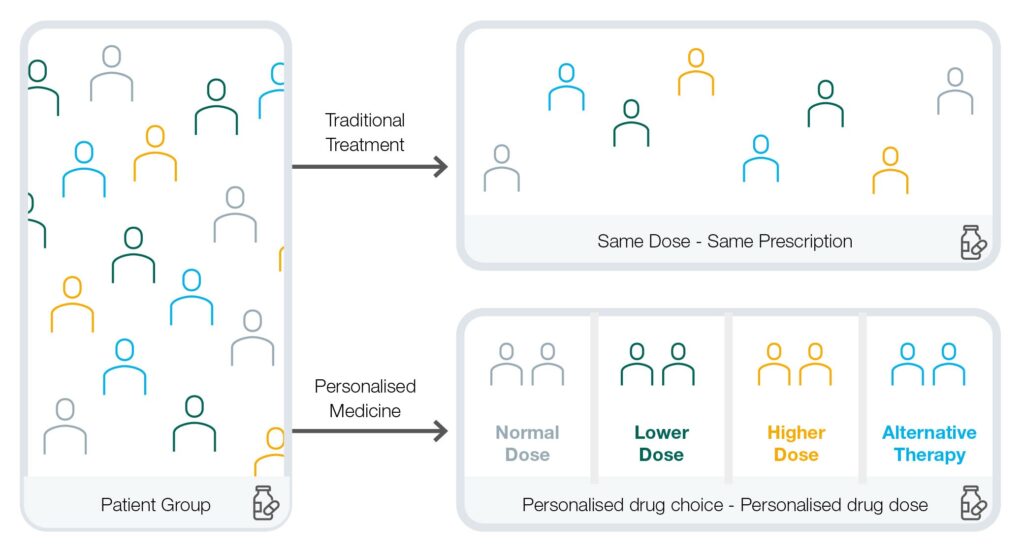
The fields of genetics and genomics have been accelerating at a rapid pace since the publication of the first human genome sequence 20 years ago, making possible the predicted shift from a disease-oriented, ‘one-size-fits-all’ model of healthcare, to an approach that is more personalised, predictive, preventative, data-driven, and cost-effective.
Genetic, or inherited, conditions have been a key element of clinical care for several decades, including rare diseases like cystic fibrosis. Genomic medicine is emerging to harness cutting-edge technologies and the large amount of genomic information generated from clinical and research activities to improve the diagnosis, treatment, and risk assessment of disease. This can lead to significantly improved patient and population outcomes across specialties in areas such as cancer, rare diseases, foetal medicine, chronic diseases, infectious diseases, and pharmacogenetics which is the study of how a person’s genetic makeup determines their response to drugs.
National Strategy for Accelerating Genetic and Genomic Medicine in Ireland
What’s more, the development of advanced bioinformatics tools for the analysis of genomic data is enabling increased use of personalised medicine. Personalised medicine is a fast-growing medical approach that takes into account a patient’s individual variables including their genetics, and in some cases their lifestyle, as well as other environmental factors.
To date, Ireland has made a degree of progress in developing genetic and genomic services, with examples of research and clinical excellence evident throughout the country. However, there is a need to mainstream genetic and genomic services and personalised medicine so that they become part of routine care delivery. More can be done to enable a coordinated national genetic and genomic service that can harness the power of genetic and genomic data, alongside research and innovation, to inform clinical decision making and advance personalised medicine in Ireland.
In early 2022, the Office of the Chief Clinical Officer of the Health Service Executive, in collaboration with the Department of Health, began the process of developing Ireland’s first national strategy to advance the field of genetics and genomics. After eight months of work and the invaluable contributions of more than 100 healthcare professionals, academics, patient representatives and international experts, the HSE launched the National Strategy for Accelerating Genetic and Genomic Medicine in Ireland in December 2022.
The National Strategy centres the voices of patients and families and builds upon existing good practice to communicate a singular vision for the development of a nationally led genetic and genomic medicine service. It also provides a path forward to advancing key areas of concern including data storage and testing infrastructure, workforce development and planning, and legal and ethical provisions.

Pictured above: Representatives of the National Strategy Steering Group at the launch event. Dr Mark Bale, Prof Walter Kolch, Muiris O’Connor, Prof Risteard O’Laoide, Deirdre McNamara, Prof Owen Smith, Prof Michael Gill, Prof Brendan Loftus
In late 2022, the Department of Health awarded an initial sum of €2.7 million to begin implementation of the National Strategy in 2023. This includes funding for additional to improve the coordination of patient care.
The HSE is now working toward the establishment of a new national office for genetic and genomic medicine and the recruitment of experts to take up key roles within that office. Once established, the national office will provide oversight and a standardised approach to the delivery of the genetics and genomics service as outlined in the National Strategy. This will align closely with relevant initiatives under the National Cancer Control Programme, the National Women and Infants Health Programme and the National Rare Disease Office.
Additional frontline workforce, including consultant clinical geneticists, genetic counsellors, and a new role of Genomic Resource Associates (GRA), will be recruited this year, and work will also begin on the development of a dedicated workforce plan to support the recruitment, retention, education, and career development of the current and future genetics and genomics workforce of Ireland. This plan will be aligned with broader HSE and Department of Health workforce plans and will build upon previous work, such as the HSE’s 2019 Review of the Clinical Genetics Medical Workforce in Ireland.

Pictured right: Director of CCO Strategic Programmes Deirdre McNamara, Minister for Health Stephen Donnelly, HSE Chief Clinical Officer Dr Colm Henry, and Steering Group Chair Dr Mark Bale at an October event supporting the development of the National Strategy
To enhance patient and public knowledge and understanding of genomic medicine, and to keep HSE staff and other stakeholders in the healthcare community informed and engaged throughout this time of change within the genetics and genomics service, a targeted communications programme is currently being developed. Patient and public involvement (PPI) efforts, continued from the National Strategy’s development process, are also underway in this early implementation phase.
Additionally, the development of a national test directory for genetics and genomics will be prioritised this year to enable appropriate and equitable testing. The national office will also start the process of reviewing existing genetic data storage and sharing capabilities in order to improve the country’s laboratory services. It will work closely with the Health Research Board funded Genomic Data Infrastructure project as part of Ireland’s commitment to the EU’s 1+ Million Genomes Initiative.
With all of these developments already underway, the implementation of the new National Strategy is off to a good start. The HSE will continue to work toward a future where genetic and genomic medicine can be equitably and expertly applied across the health service and throughout the lifespan of patients by healthcare professionals who are supported in that endeavour.
The National Strategy for Accelerating Genetic and Genomic Medicine in Ireland is available at www.hse.ie > About Us > Strategic Programmes Office Overview.
Genetics:
The study of genes, genetic variation, and heredity in living organisms.
Genomic medicine:
The use of genomic information and technologies to determine disease risk and predisposition, diagnosis and prognosis, and the selection and prioritisation of therapeutic options.
Personalised medicine:
Medicine targeted towards an individual or group of individuals, which uses knowledge of genetic, environmental and lifestyle factors to determine suitable methods of prevention, diagnosis, and treatment of disease.
Pharmacogenetics:
The study of how an individual’s entire genetic makeup determines the body’s response to drugs. Genome-guided prescribing can help to avoid severe and life-threatening adverse reactions to pharmaceuticals, determine drug selection and dosage to maximise the therapeutic benefit and minimise side effects, improve patients’ compliance with treatment by targeting the right patient with the right drug (thereby improving patient experience), and reducing the time, cost, and failure rate of pharmaceutical clinical trials.

Prof. Eileen Treacy, Clinical Lead, National Rare Diseases Office
Genetics and genomics has transformed our understanding of disease and our ability to deliver care that is ‘personalised’: that improves the diagnosis, monitoring, and treatment of rare diseases, markedly shortening the ‘diagnostic odyssey’. Inborn errors of metabolism (e.g. PKU, Familial Hypercholesterolaemia) are great examples.
An individual in Ireland first came to medical attention as a child with severe muscle pain, weakness, and kidney abnormalities associated with a markedly raised muscle damage enzyme (CPK). A muscle biopsy showed non-specific abnormalities in the mitochondria (‘powerhouses of the cell’).
She subsequently had regular episodes of marked muscle weakness and pain when exercising or with ongoing intercurrent illness and ‘cyclical vomiting’. A family member had a similar presentation.
In her twenties, she delivered her first healthy child by caesarean (C-section). After the delivery, she spent twenty days in her regional hospital due to prolonged weakness of her legs. A few years later, she delivered a second infant again by C-section and the same leg weakness occurred with a prolonged period in hospital. In 2021, next-generation sequencing (molecular genetic) panels were organised at the National Adult Metabolic Centre (over twenty years after the initial presentation). It was confirmed that she had inherited two copies of a disease-causing variant in the CPT2 gene causing deficiency of the enzyme carnitine palmitoyl transferase (CPT2). This results in failure of transport of carnitine (transport nutrient) into the cell for the use of lipids (fat) for energy.
Following the confirmatory diagnosis, she had a recent third pregnancy. Despite a preventative metabolic treatment protocol in place, she again developed profound weakness of her lower limbs following the repeat C-section and bupivacaine use as the local anaesthetic. We proposed that bupivacaine might further block the abnormal carnitine transport contributing to the prolonged weakness. High dose carnitine was commenced which greatly improved her strength, an example of ‘personalised medicine’ following the confirmatory genomic diagnosis.

Karen Morgan, Patient Representative and Steering Group member, Deirdre McNamara, Director of CCO Strategic Programmes, Prof Owen Smith.

Prof. Karen A Cadoo. Consultant Cancer Geneticist and Medical. Oncologist
Ovarian cancer provides a powerful example of the value to integrating routine genetic testing into cancer care. When women are diagnosed with ovarian cancer, they are offered genetic testing. For some women a BRCA1/2 pathogenic variant (or mutation) is the reason that they unfortunately developed ovarian cancer. Finding out they have a BRCA pathogenic variant is difficult for a patient and her family. However, it gives us key information for a woman’s own cancer care and opportunity to reduce future cancer risk.
We now know that women who have a BRCA-associated ovarian cancer have better prognosis. We can also use this information to predict the potential for response to therapy, both chemotherapy and new drugs, such as poly ADP ribose polymerase (PARP) inhibitors.
PARP inhibitors have been revolutionary for women with BRCA associated ovarian cancer, doubling the number of women who are disease free five years after their diagnosis. We hope that some of these women will be cured of their ovarian cancer, and that is a huge breakthrough.
We also have the opportunity to intervene in their future cancer risk through intensive breast cancer surveillance. Critically, we have huge opportunity for their families. Genetic testing can identify family members who have increased cancer risk and initiate management strategies.
We can utilise genetic testing to achieve our goals in cancer control nationally. Genetic testing can help to reduce the burden of cancer in our population through prevention and early detection. We can also reduce the burden of cancer care through targeted therapy approaches.
We have made huge progress which has led to expansion in the role and demand for genetic testing in cancer care. It is fantastic to see the opportunity to put infrastructure in place to deliver equitable and timely access to genetic testing for our population in Ireland.
Dr Mark Bale at the launch event for the National Strategy
Dr Mark Bale

Chair of the National Genetics and Genomics Steering Group
Highlighted Articles