Home » Aquipta 10mg and 60mg tablets — new treatment for migraine
Aquipta tablets containing 10mg or 60mg of atogepant, are indicated for the prophylaxis of migraine in adults who have at least four migraine days per month. They were added to the High Tech Scheme in February 2025.
The recommended dose is 60mg atogepant once daily and it can be taken with or without meals. The tablets should be swallowed whole and should not be split, crushed, or chewed.
Strong CYP3A4 inhibitors (for example, ketoconazole, itraconazole, clarithromycin, ritonavir) and strong organic anion transporting polypeptide (OATP) inhibitors (for example, rifampicin, ciclosporin, ritonavir) can significantly increase systemic exposure to atogepant. If co-administration with strong CYP3A4 inhibitors or strong OATP inhibitors is required, the daily dose needs to be reduced to 10mg daily.
Aquipta is a calcitonin gene-related peptide (CGRP) antagonist.
The most commonly reported adverse drug reactions were nausea (9 per cent), constipation (8 per cent) and fatigue/somnolence (5 per cent). Most of the reactions were mild or moderate in severity. The adverse reaction that most commonly led to discontinuation was nausea (0.4 per cent).
Aquipta was first authorised in the EU in August 2023 and was added to the High Tech Scheme for February 2025. This medicine is subject to additional monitoring (black triangle). This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
This medicinal product does not require any special storage conditions.
High Tech Numbers:
Aquipta 10mg Tablets x 28: 89382
Aquipta 60mg Tablets x 28: 89383
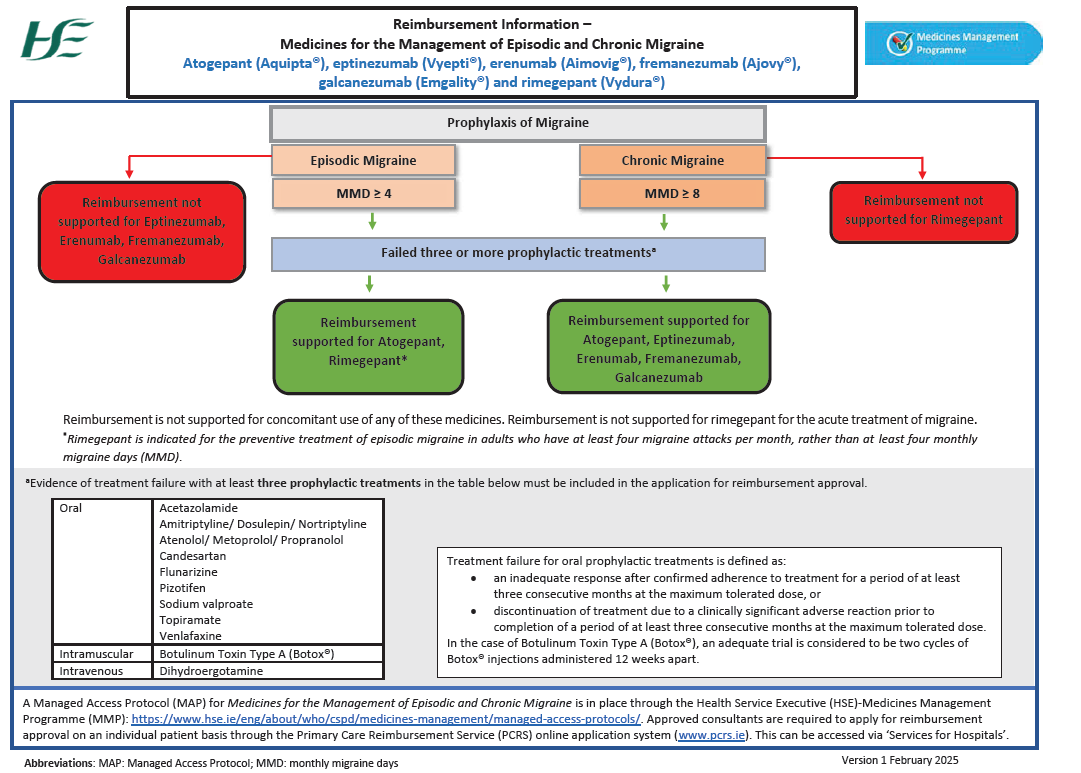
A Managed Access Protocol (MAP) is in place for medicines for the management of episodic and chronic migraine, which outlines the criteria that must be satisfied in order for a patient to be recommended for reimbursement of atogepant (Aquipta), erenumab (Aimovig), fremanezumab (Ajovy), galcanezumab (Emgality) or rimegepant (Vydura), under the High Tech Arrangement, or eptinezumab (Vyepti), under Hospital Pricing Approval.
Applications for reimbursement approval will only be considered from consultant neurologists who have agreed to the terms of the MAP and have been approved by the HSE. The approved consultant must apply for individual reimbursement approval for each patient. For patients with a diagnosis of episodic migraine, clinicians will be required to confirm that the number of monthly migraine days the patient experienced in the one month prior to the date of application is greater than, or equal to, four.
For patients with a diagnosis of chronic migraine, clinicians will be required to confirm that the number of monthly migraine days the patient experienced in the three months prior to the date of application is greater than or equal to eight.
Evidence of treatment failure with at least three prophylactic treatments must be included in the application. Ongoing reimbursement approval is conditional on a reduction in the frequency of migraine of at least 50 per cent in episodic migraine and at least 30 per cent in chronic migraine.
The MAP for Migraine is available on the HSE website at hse.ie/eng/about/who/cspd > Medicines Management Programme > Managed Access Protocols > Migraine.
Figure 1: HSE Managed Access Protocol for Medicines for the Management of Episodic and Chronic Migraine Summary
Source: HSE, available at hse.ie/eng/about/who/cspd > Medicines Management Programme > Managed Access Protocols > Migraine.
Tara Kelly

Medicines Information Pharmacist, IPU
Highlighted Articles