Home » What is Open Disclosure?
The recently enacted Patient Safety Act aims to embed openness and transparency in healthcare settings, including in private healthcare settings. In this article, the IPU’s Head of Professional Services (Acting), Sinéad McCool, provides an overview of how it will impact community pharmacy.
Open Disclosure is an open, honest, empathic, and timely approach to communicating with patients and their families when things go wrong in healthcare.
The Patient Safety (Notifiable Incidents and Open Disclosure) Act 2023 was enacted in September 2024. The legalisation aims to embed openness and transparency in healthcare settings in Ireland, including in private healthcare. The Act sets out the legal framework for the reporting and the management of incidents for mandatory open disclosure and includes a list of specific patient safety incidents that must be reported. Noticeable incident must be reported to the Health Information and Quality Authority, Chief Inspector of Social Services and the Mental Health Commission.
The Department of Health has also developed a Patient Safety Act guidance document to assist stakeholders in understanding the provisions of the Act.
There are 13 specific notifiable incidents which are explicitly bound by legislation in how they are responded to and are subject to mandatory reporting. These include incidents involving surgery, use of blood products, or an unexpected death in hospital.
Notifiable Incident number 8 is described as a “Patient death associated with a medication error and the death was unintended and unanticipated as it did not arise from or was a consequence of (or wholly attributable to) the illness of the patient or an underlying condition of the patient.”
A ‘Designated Person’ is someone who has been assigned to act as a liaison between the health services provider and the patient or relevant person (or both of them) in relation to the open disclosure of the notifiable incident and in respect of a request for clarification under section 23.
A ‘designated person’ is not a permanent role. This person is only required when open disclosure of a particular notifiable incident is necessary. In the case of an independent pharmacy, it is likely that this will be the Superintendent Pharmacist. Again, it is important to note that this requirement is in respect to open disclosure of ‘notifiable incidents’.
The National Patient Safety Office (NPSO) of the Department of Health developed the National Open Disclosure Framework. This policy document is to aid in the implementation of the new patient safety and open disclosure legislation.
The National Open Disclosure Framework aims to promote a clear and consistent approach to open communication, by health and social care service providers and other organisations where appropriate, with patients/service users and any relevant support person, following a patient safety incident or an adverse event. It aims to provide a unified and consistent approach to open disclosure across the entire health sector:
The Framework is designed to be used in the development, or upgrading, of an organisation’s internal policies, processes, education and training, and practices regarding patient safety incidents and adverse events.
The Framework ensures that the rights of all patients/service users and health and social care staff involved in and affected by patient safety incidents and adverse events are met and respected.
The Framework provides six overarching principles that are expected to be used to underpin open disclosure in practice, including in pharmacy practice (see Table 1).
Table 1: Principles of the National Open Disclosure Framework
Principle 1: Open, Honest, Compassionate, and Timely Communication
Principle 2: Patient/Service User and Support Person’s Entitlement in Open Disclosure
Principle 3: Supporting Health and Social Care Staff
Principle 4: Promoting a Culture of Open Disclosure
Principle 5: Open Disclosure for Improving Policy and Practise in Health and Social Care
Principle 6: Clinical and Corporate Governance for Open Disclosure
A patient safety incident, in relation to the provision of a health service to a patient by a health services provider, means “an incident which occurs during the course of the provision of a health service”, which:
Therefore, a patient safety incident includes harm events, no harm events, and near miss events.
An adverse event is an incident which resulted in harm that may or may not be the result of an error.
Open disclosure champions must be identified and appointed by health and social care service providers to lead and promote open disclosure policy, education, and training, and monitor practice.
Any pharmacy staff member can be nominated as a champion, for example a pharmacist/owner, pharmacy technician, manager or other staff.
For pharmacists working in patient facing roles, they must:
For pharmacists undertaking governance roles and pharmacy owners, they must ensure robust policies and SOPs are in place which underpin open disclosure practices and support a culture of open disclosure in the pharmacy. This may include:
There may be additional annual reporting requirements on open disclosure to the Department of Health for community pharmacies, dependant on the number of employees in the community pharmacy, or chain of community pharmacies. The IPU are awaiting further information on this from the Department of Health.
The incident should be reported as per incident management policy and SOPs implemented at the pharmacy. Identify the category level the incident falls into. For example, the Framework notes that different approaches may be taken to open disclosure, depending on whether a ‘low’ or ‘high’ level response is required. The level of response required will be defined by the degree of harm the patient has experienced, the level of additional interventions or treatments required because of this harm, and the expectations of the patient and their support person. Responses may vary from one open disclosure meeting to a number of meetings.
Examples of where ‘lower’ level responses might be required include:
Firstly, it is imperative to appropriately manage the situation in the immediate aftermath of the patient safety incident or adverse event to avoid any possible or further potential harm to the patient.
The incident should be reported as per incident management SOPs implemented at the pharmacy which reflects the requirements of the Patient Safety Act. As outlined policy and SOPs should include:
A notifiable incident must ALSO be reported via the National Incident Management System (NIMS) to HIQA. For community pharmacists there is a specific NIMS portal to be used for reporting. There is further information on the NIMS on the HIQA website including specific guidance for health service providers on how to notify HIQA of a notifiable incident under the Patient Safety Act.
An open disclosure meeting must be held with the patient and/or their representatives. A designated person should lead on this meeting. The Framework provides a useful checklist of issues to consider prior to this meeting.
When a patient safety incident or adverse event occurs, patients/service user and their support person want:
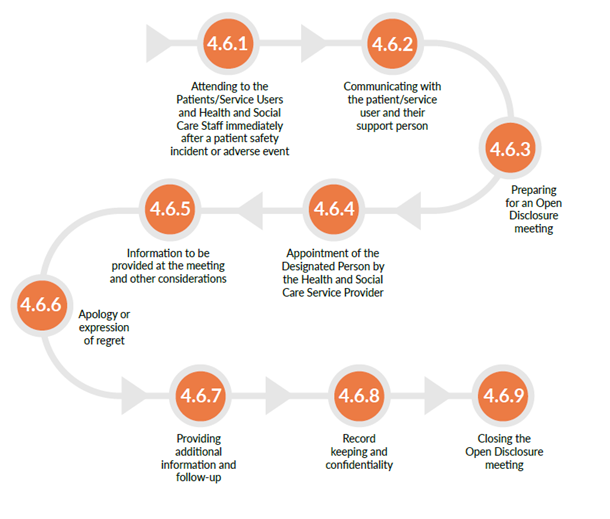
Image 1: Open Disclosure Process
Sinéad McCool MPSI

Head of Professional Services (Acting), IPU
Highlighted Articles