Home » Reimbursement restrictions for items dispensed under the Community Drug Schemes

In the context of healthcare and pharmacy services provided by pharmacists under the community pharmacy contractual agreement, it is the responsibility of the PCRS, to review and validate reimbursement claims, ensuring that the patient is eligible for the supplied medication and that the quantities requested do not exceed the ‘reasonable needs’ of the patients being treated.
Under the community drug schemes, the PCRS will only reimburse a pharmacy for a one-month supply of medications. However, to allow for the shortfall, due to pack sizes, in a 12-month period, pharmacies are allowed to dispense an extra pack of medication. So, if a pack was on a 28-day cycle, pharmacists can supply 13 packs in a 12-month period. The packaging of a particular item will determine whether the thirteenth dispensing is required by the patient in a 12-month period.
Standard practice for PCRS is to reimburse a pharmacy up to the maximum quantity as specified in the Summary of Product Characteristics (SmPC) for a medicine required by a patient per calendar month. These limits are specified within the pharmacy contract, which states:
“4(2) ‘Where a prescription is issued in respect of a quantity of a medicine which does not conform to the amount in the pack presentation of the product which is available on the market, the pharmacist, in the exercise of his/her professional judgement and discretion, may supply the quantity in the nearest pack presentation provided that the quantity so supplied is not normally greater that one month’s supply for the patient concerned.”
As PCRS does not publish what it deems as acceptable monthly usage for all reimbursed items, the SmPC should be used as a guide in setting the maximum quantities to supply to any one patient. In exceptional circumstances where quantities are dispensed outside the recommended allowances, pharmacists should check with the PCRS if the prescribed quantity will be reimbursed in full before any additional supplies are made to a patient. Additional documentation may be requested to support these claim types.
The Health Pricing and Supply of Medical Goods Act 2013, implemented various measures to generate cost savings for the HSE, for patients or both, and to establish mechanisms to set reimbursed prices for items dispensed under the community drug schemes. Implementing this legislation with the associated increase in PCRS circulars has contributed to the increasing administrative burden experienced within the community pharmacy practice.
In addition to the establishment of an interchangeable medicine list and reference pricing, this legislation enabled the HSE to impose conditions on the supply of some reimbursable items, including:
The Medicines Management Programme has undertaken a number of initiatives aimed at enhancing evidence-based and cost-effective prescribing nationally. Some examples include:
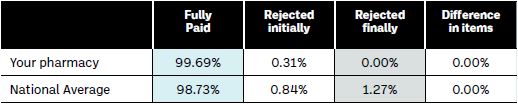
Pharmacists will be able to determine the level of rejected claims for their pharmacy by the information in the DPS/LTI summary listing booklets provided by the PCRS. The final page of this document provides valuable information on the percentage of claims that have been fully paid, as well as those initially rejected and finally rejected, for the individual pharmacy in comparison to the national average (see Table 1 below).
Table 1: Summary Listing Information| Fully paid | Rejected initially | Rejected finally | Difference in items | |
| Your pharmacy | 99.69% | 0.31% | 0.00% | 0.00% |
| National Average | 98.73% | 0.84% | 1.27% | 0.00% |
Once the electronic claim file has been transmitted at the end of the month to the PCRS, the exceptions file will be available for download within 24 hours. Downloading the early exception file and resubmitting your rejects helps pharmacists to minimise any financial loss from the end of month process, before the rejected claims move to become final rejects. It is important to build in time into your dispensary operations to download these and resend them within the claim period. The full claim reject listing will outline any items/forms that the PCRS has identified as rejected claims.
Occasionally, the same item can have more than one type of restriction applied e.g. Saxenda®. Saxenda® supplied under the community drug schemes is restricted to specific patient categories that meet the criteria as per PCRS Circular 002/23 for a limited time and the reimbursed quantity of Saxenda, it is limited to just one box per month.
Examples of restricted items:
As part of cost-containment measures and ongoing evaluations, the PCRS has set limits for high-cost items and/or items with ambiguous dosages. The PCRS employ clinical guidance to manage reimbursement quantities for these items. Rather than an outright reject, the PCRS may reduce the payment to the pharmacy based on maximum allowed quantities. Therefore, it is crucial for pharmacists to exercise scrutiny when reviewing paid claims to identify any such adjustments, the PCRS produce a monthly XML claim file that will help identify these discrepancies.
A wide range of conditions may require nutritional support, but dispensing ONS on a repeat GMS prescription (GMR) is not permitted for reimbursement purposes. Standard Oral Nutritional Supplements (ONS) that do not necessitate reimbursement approval prior to supplying are included in the first-line prescribing list for adults living in the community, established in 2019. However, for non first-line ONS (list B), prior reimbursement approval is mandatory before dispensing under the community drug schemes. The comprehensive overview of List B is available on the Medicines Management Programme webpage for Oral Nutritional Supplements. The approval for these items remains valid for up to six months and is prescriber-initiated. Continuing ONS when treatment objectives have not been achieved is not good practice, so the approval is subject to renewal.
“ If pharmacists supply quantities beyond these limits to patients under the community drug scheme, they risk financial losses and will not receive reimbursement for the total number of supplied items.”
The recommended blood glucose test strips (BGTS) allowances are recommended in HSE Circular 011/16 which outlines the restrictions for differing diabetic conditions as follows:
Women diagnosed with gestational diabetes who have current GMS eligibility must have their physicians register them online for full access to diabetic strips without a threshold. Patients with gestational diabetes are not entitled to apply for LTI eligibility based on their condition. For patients with DPS eligibility who have been diagnosed with gestational diabetes a maximum of 250 strips a month will be reimbursed under this scheme, no additional registration is required for these DPS patients.
The HSE has established managed access protocols to address the clinical and financial implications related to specific high-cost medicines and devices. This is a restricted reimbursement application system that not only considers patients’ eligibility but also serves to manage the impact of these products on healthcare budgets. By implementing this system, the HSE aims to ensure that the reimbursement of these items is carefully regulated and aligned with established criteria and guidelines. Pharmacists can verify approval using the ‘Eligibility Confirmation’ feature within the Pharmacy Application Suite under ‘Patient Specific Arrangements’. Claims submitted for patients who are not approved will not be paid.
Due to the budget impact associated with continuous and flash glucose monitoring systems, PCRS has a reimbursement application system in place. The reimbursement application can be made by a Consultant Endocrinologist responsible for initiating treatment through the PCRS Hospital Application Suite.
If a patient reports a fault with an FGM or CGM product, a replacement must be sought from the manufacturer and not through a repeat dispensing.
To support pharmacists in identifying reimbursement issues before dispensing, we have compiled a comprehensive Restricted Reimbursement Guide and a Summary of Items with Restrictions Guide outlining the current known reimbursement restrictions. IPU members can locate these documents at www.ipu.ie > Contract > Reimbursement.
Increasing awareness and facilitating appropriate management of reimbursement considerations within a pharmacy practice will reduce rejected claims, if you need more support please contact contract@ipu.ie.
[1] PCRS Rejects Report for August 2022 with post corrections up to 9 months after
Zrinka Bulj

Claims Analyst, IPU
Highlighted Articles