Home » Psoriatic arthritis: will disease prevention trials soon become possible?
A new European study has been launched, which aims to recruit 25,000 psoriasis patients online from across Europe, with 2,000 of these in Ireland. The study, HPOS, will then follow them for the development of psoriatric arthritis. In this article, the HPOS team provide further information on both the study itself, and treatment options for both psoriasis and psoriatic arthritis.

According to the International Federation of Psoriatic Disease Associations (IFPA), cutaneous psoriasis (PsO) affects around 60 million people worldwide, while in Ireland at least 73,000 individuals suffer from PsO. PsO is a chronic, genetic, inflammatory skin condition, affecting both sexes equally, and there is currently no cure. PsO may be triggered by environmental factors, including infections, smoking, and stress. Five types of PsO have been identified (see Figure 1):
Figure 1: Types of Psoriasis
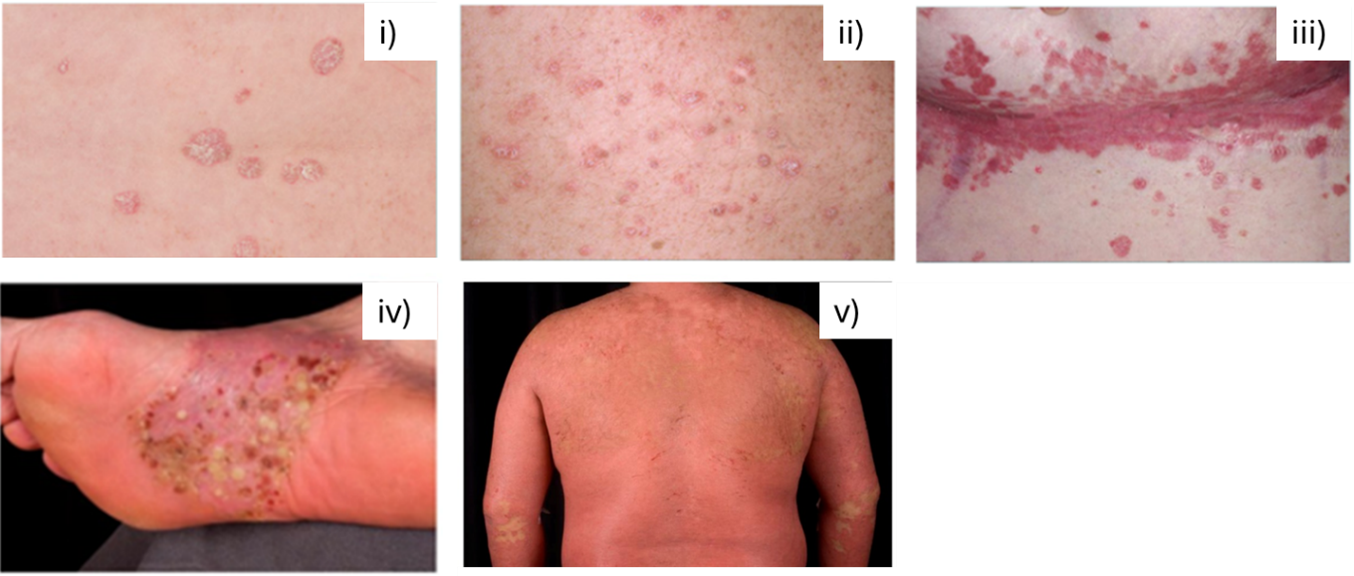
(Sources: Irish Skin Foundation, Psoriasis booklet 2018; Rendon & Schäkel, 2019).
Plaque psoriasis accounts for 90 per cent of PsO cases and generally presents as itchy, scaly, thick skin plaques; occurring in symmetrical locations on the body, for example on elbows, knees, the back of the hands, finger nails or the scalp.
While PsO appears as a skin condition, it is well known that PsO is a systemic disease that can be associated with many comorbidities. There is an increased risk for the metabolic syndrome and of cardiovascular diseases in PsO, but PsO also commonly affects mental health, with reduced quality of life and higher rates of depression.
PsO also increases the risk for the development of an associated form of arthritis, called Psoriatic Arthritis (PsA), with about 30 per cent of PsO patients developing PsA. PsA symptoms include joint pain, stiffness, loss in functionality, lack of sleep and general fatigue. Generally, PsA manifests as peripheral arthritis — affecting the large joints of arms, legs, elbows, wrists, knees and ankles; spondylitis — affecting the spine; enthesitis — where tendons and ligaments attach to the bone, such as the Archilles tendon; or dactylitis — causing painful swelling of the fingers and toes.
A PsA diagnosis is usually made when inflammatory musculoskeletal involvement is detected in the presence of cutaneous PsO and in the absence of rheumatoid factor and anti-Cyclic Citrullinated Peptide antibody. A diagnosis is often delayed due to the lack of awareness about PsA, and its consequences, by the patient but also the medical professional. Misdiagnosis of PsA can also lead to delay in effective treatment, since therapies for other forms of arthritis are not successful in treating PsA.
Figure 2: Treatment goals
For those who suffer with mild PsO this may be managed with topical prescription therapies often containing tar, salicylic acid, steroids, Vitamin D analogues or a combination of these agents, depending on the affected site. Over-the-counter treatments such as emollients are also important to moisturise the skin and help with the dryness, scaliness and itch associated with PsO. Urea-containing moisturisers can be particularly useful for thick plaques. Shampoos containing ketoconazole or tar can help with inflammation of the scalp, while salicylic acid shampoos can help reduce scaling. It is very important to avoid scratching, picking or trauma to PsO lesions as this can result in more PsO development at these sites (known as the Koebner phenomenon).
Phototherapy, or light therapy is also used to treat those with mild-moderate PsO and is particularly effective in managing an acute eruption of guttate PsO. This involves exposing the skin to a very specific and safe range of narrowband ultraviolet-B light on a regular basis (usually three times a week) under medical supervision by a Consultant Dermatologist. It is typically successful in clearing PsO in the majority of patients. The duration of clearance is very variable — from a few weeks to several years in some patients. This is a very safe form of therapy and can also be used during pregnancy and in children. Gentle sun exposure during summer months can also help PsO but it is important to avoid sunburn which can induce PsO plaques.
PsO can have a considerable impact on a patient’s mental health, relationships and intimacy. Patients with greater than 10 per cent body surface involvement of PsO are considered to have more severe disease. Often when patients have this level of PsO, topical treatments are poorly effective and cumbersome to use. Alternatively, phototherapy, oral systemic medications or injectable biologic agents can be used. Systemic oral agents include traditional agents such as methotrexate, cyclosporine, fumaric acid esters and the newer small molecules, apremilast — a PDE4 inhibitor, and deucravacitinib — an oral selective TYK2 inhibitor.
Biologic drugs are a newer category of drugs given by injection to target more specific parts of the immune system and have revolutionised the treatment of PsO. Now patients with PsO can expect to be completely clear or almost clear their disease. Several categories of biologic drugs exist which block different inflammatory pathways that cause PsO — the TNF inhibitors, the IL-12/23 inhibitor, the IL-17 inhibitors, and the IL-23 inhibitors. Individualising treatment with consideration of patients’ needs and individual preferences should be central to the management of PsO. The future for patients with severe PsO is bright.
Some over-the-counter (OTC) medications can be helpful in managing symptoms related to arthritis. Analgesics such as paracetamol, taken as required, or on a regular basis up to four-times per day, can help reduce the pain associated with musculoskeletal inflammatory disease. OTC non-steroidal anti-inflammatory drugs (NSAIDs) may also reduce inflammatory symptoms but should be taken with caution in patients with underlying renal or hepatic disease, should not be combined with anti-coagulants, and should be avoided in those at risk of peptic ulcer disease. NSAIDs are really a short-term option while awaiting the initiation of more definitive disease-modifying anti-rheumatic drug (DMARD) treatment.
DMARDs in PsA are broken down into three categories: standard synthetic DMARDs, targeted synthetic (TS)-DMARDs and biologic (b)-DMARDs. Standard synthetic DMARDs include traditional agents such as methotrexate and leflunomide. TS-DMARDs include apremilast and a number of Janus Kinase (JAK) inhibitors such as Tofacitinib and Upadacitinib. The list of b-DMARDs is the same as for skin PsO but efficacy for the skin disease features is generally better than for the musculoskeletal features.
While there has been substantial progress in terms of available and effective treatments for PsA, there remains a significant unmet need for the development of personalised treatment approaches based on an individual patient’s characteristics.
Insert Figure 3: The Burden of Psoriasis

(Extract from The Burden of Psoriasis Epidemiology, Quality of Life, Co-morbidities and Treatment Goals, L. Barnes, B. Kirby, O. Fitzgerald, A.M. Tobin (eds.), (Novartis Ireland, J.E. Cairnes School of Business and Economics NUI Galway, and the Irish Skin Foundation, 2015). Available at irishskin.ie.
Design note: available at https://irishskin.ie/wp-content/uploads/2023/04/Burden_of_Psoriasis_Report_final.pdf, p. 8
As part of HIPPOCRATES (HIPPOCRATES consortium, see hippocrates-imi.eu), a new online study has been established, the HIPPOCRATES Prospective Observational Study (HPOS). HPOS aims to monitor the development and progression of PsA in a population with cutaneous PsO, hoping to find ways to prevent the development of PsA in the future. Participants, who are over 18 years of age and diagnosed with PsO, but not with PsA, will be monitored for three years via six-monthly questionnaires. Participants who are developing symptoms of PsA are informed about their risks, receive a recommendation to seek an appropriate medical assessment, and they might be requested to take part in a remote blood sampling study for biomarker analysis. While more than 1,700 Irish people with Pso have already registered to participate in HPOS, the study is still recruiting. For more information, see hpos.study.
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 101007757. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
“Cutaneous Psoriasis (PsO) can have a considerable impact on a patient’s mental health, relationships and intimacy.”
Dr Teresa Grohmann PhD / Professor Caitriona Ryan MD / Professor Stephen Pennington PhD / Professor Oliver FitzGerald MD

Highlighted Articles