Home » Original Pack Dispensing
Original Pack Dispensing (OPD) is an evolving practice in the pharmacy sector aimed at improving both efficiency and patient safety. Dispensing medications in their original manufacturer’s packaging rather than repackaging them into smaller quantities will also facilitate better stock control and adherence to FMD legislation.
Recent legislative changes in the UK in 2023 have given flexibility around the quantity prescribed versus quantity dispensed, and so has further facilitated the adoption of Original Pack Dispensing. The legislation allows pharmacists to dispense up to 10 per cent more, or 10 per cent less, than the quantity prescribed if it enables the medication to be supplied in its original packaging. For instance, if a prescription calls for 28 tablets, but the original pack contains 30 tablets, the pharmacist can dispense the full pack.
It also brought in new rules requiring pharmacists to dispense all licensed medicines containing valproate in the manufacturer’s original full pack.
Most European countries (such as Belgium, Austria, Sweden, Italy, Portugal and France) original pack dispense all medications, both acute and chronic. As it is long-standing practice in these countries, they have various pack sizes of each medication to enable them to dispense an original pack that is close in size to that prescribed.
The model of supply in original packs was clearly communicated to all our stakeholders in the IPU White Paper Key Enablers for a Sustainable Pharmacy Model.

Figure 1: Cut tablet strips
Original pack dispensing (where appropriate) would have numerous benefits for patients and pharmacy teams including:
Some potential barriers to implementation would need to be considered:
The PSI’s Good Dispensing Practice — High Tech Scheme states that High Tech medicines, where possible, should be dispensed in the manufacturer’s original pack.
The HSE Primary Care Eligibility and Reimbursement Service’s (PCRS) Information and Administrative Arrangements for Pharmacists states that; “Where the quantity of a preparation prescribed does not correspond with an original pack size and it is not feasible to supply the exact amount prescribed the pharmacist may, in the exercise of his professional judgement and bearing in mind the nature of the product and his statutory obligations, supply the original pack size nearest to the quantity ordered.”
Figure 3: Tamper-proof seal
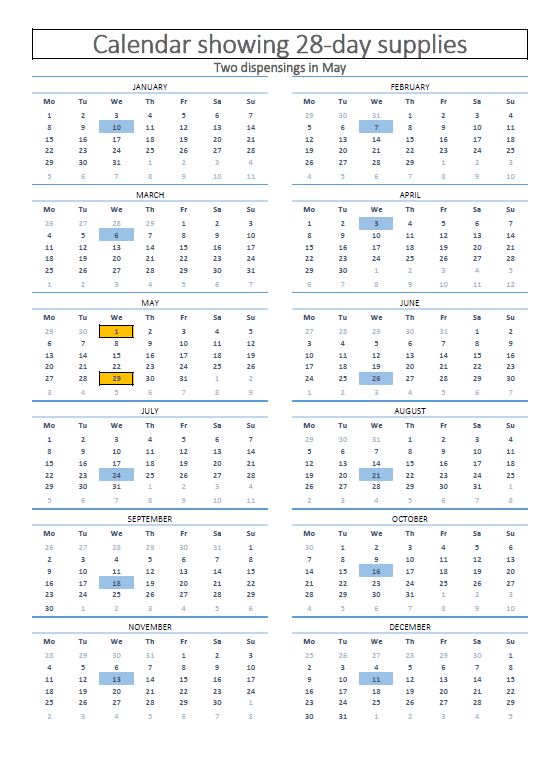
It is also important to discuss the options for a balancing supply of certain medications based on patient need. Where a 28-day supply of medication is routinely supplied to an individual or family under the community drug schemes, the HSE-PCRS allow for a balancing supply of medication (for example, a 13th dispensing of 28 days’ worth of medication in a 12-month period), where no co-payment applies. This is to ensure that no individual or family makes more than 12 payments in a calendar year. A printed calendar to show patients 13 dispensing’s over the year with 12 community drug scheme payments may provide reassurance to patients of how this would work in practice. Where any additional cost may rest with the patient, this should be clearly explained.
The Association of the British Pharmaceutical Industry circulated advice to all its members in February 1986 that chronic treatment packs should contain treatment for 28 days, while short-term treatment packs should contain the quantity required to meet the manufacturer’s recommendations for a course of treatment. This would avoid the breaking of bulk to meet prescriptions.
In this year’s IPU AGM, a motion calling for a similar policy was passed which said:
“That this AGM calls on the Department of Health to implement a policy of a standard pack size for medications in Ireland. This will facilitate patient compliance and will reduce administrative workload in pharmacies and reduce risk within the dispensing process.”
A move to standard pack sizes would make the transition to original pack dispensing easier but is not something which has been implemented in Ireland to date. Given the current high level of medicine shortages, we would not want to insist on implementation of a measure that may impact medicine supply further.
Pharmacists should educate patients about original pack dispensing, explaining why they might receive a slightly different quantity than prescribed and reassuring them about the safety and benefits of this practice. Clear communication helps prevent confusion and builds trust. Patients must be informed of the proposed change in practice well in advance of it occurring. This could take the form of:

Informative material should be available to support patients in making an informed decision about moving to such a system. Give adequate time to having conversations with patients about the rationale, impacts and benefits of such a move, and allow patients time to ask questions. It might be beneficial to identify patients who previously insisted on 30-day or 31-day supplies of medication and for the pharmacist to take extra time to discuss the change in policy with them. It is important to listen to patients and respect their views about their health and medicines and to get their agreement to move to original pack dispensing. It may be the case that not all patients agree to move to the new system.
Those in governance roles in pharmacies should ensure that the practice observed at the pharmacy is compliant with legislation, PSI guidance and HSE-PCRS reimbursement requirements.
Pharmacists should use their professional judgment to determine if original pack dispensing is appropriate for each patient. This includes considering the patient’s ability to manage their medication, the potential impact on treatment outcomes, and ensuring that the slight variance in quantity does not negatively affect the patient’s clinical treatment.
Pharmacists should make a professional determination as to the suitability of original pack dispensing on a case-by-case basis. In general, supply of medication for the treatment of a chronic condition is more suitable for original pack dispensing. The following types of medications could be considered for original pack dispensing:
Pharmacists should record any deviations from the prescribed quantity and ensure that all relevant information is communicated to the patient and documented in their records. This will allow for auditing of the records to see how the policy is being implemented and what is working well or not.
Encourage patients and pharmacy team members to provide feedback on their experience with original pack dispensing. This can help the pharmacy understand patient concerns and foster a continued improvement behaviour within the pharmacy team.
Supporting materials such as an Original Pack Dispensing policy document, a poster, a patient leaflet, a patient information sheet, a calendar showing an example of 13 dispensing’s in a year and a template letter to inform local prescribers are available under the SMART Pharmacy section of the IPU website, ipu.ie > SMART Pharmacy.

“We recently introduced original pack dispensing in all our stores and it has been a big success. From busier stores to quieter stores, there has been no significant feedback of resistance to the change.” – Áine McCabe, McCabe’s Pharmacies
References available on request
Ciaran Mulligan MPSI

Interim Contracts Manager, IPU
Highlighted Articles