Home » IPU Medicines Information Pharmacist
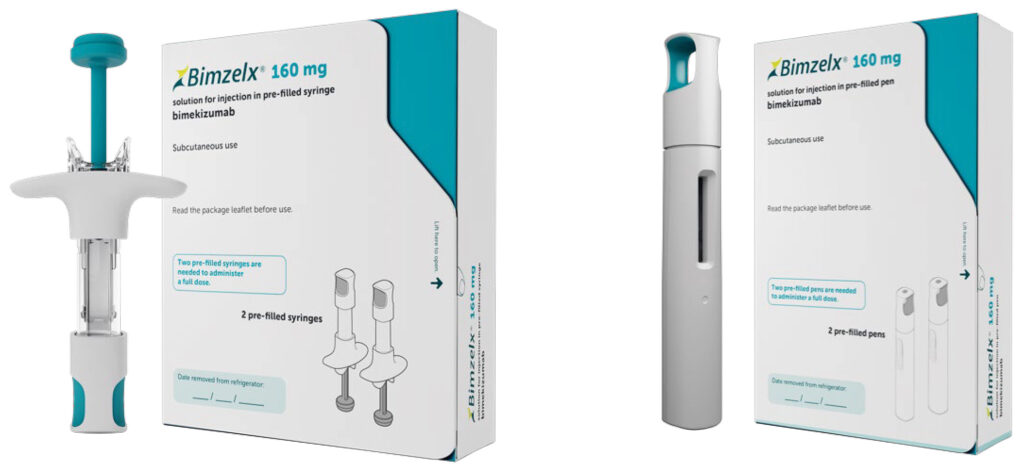
Bimzelx 160mg pre-filled syringe and pre-filled pen are now available on the High-Tech Medicine Scheme for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
Each pre-filled device contains 160mg of bimekizumab in 1ml. Bimekizumab, a new active substance, is a humanised IgG1 monoclonal antibody produced in a genetically engineered Chinese hamster ovary (CHO) cell line by recombinant DNA technology. Bimzelx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of plaque psoriasis.
Bimekizumab is a humanised IgG1/κ monoclonal antibody that selectively binds with high affinity to IL-17A, IL-17F and IL-17AF cytokines, blocking their interaction with the IL-17RA/IL-17RC receptor complex. Elevated concentrations of IL-17A and IL-17F have been implicated in the pathogenesis of several immune-mediated inflammatory diseases including plaque psoriasis. Bimekizumab inhibits these proinflammatory cytokines, resulting in the normalization of skin inflammation and therefore improvement in clinical symptoms associated with psoriasis.
The recommended dose for adult patients with plaque psoriasis is 320mg (given as two subcutaneous injections of 160mg each), at week 0, 4, 8, 12, 16 and every 8 weeks thereafter. Consideration should be given to discontinuing treatment in patients who have shown no improvement after 16 weeks. Suitable areas for injection include the thigh, abdomen, and upper arm. Injection sites should be rotated, and injections should not be given into psoriasis plaques or areas where the skin is tender, bruised, erythematous, or indurated. The pre-filled syringe or pre-filled pen must not be shaken. After proper training in subcutaneous injection technique, patients may self-inject Bimzelx if their consultant determines that it is appropriate, and with medical follow-up, as necessary. Patients should be instructed to inject the full amount of Bimzelx according to the instructions for use provided in the package leaflet.
The most commonly reported adverse reactions were infections, neutropenia, hypersensitivity, headache, inflammatory bowel disease, dermatitis, eczema, acne, injection site reactions, and fatigue.
High Tech Numbers:
Bimzelx 160mg solution for injection pre-filled pen 2 pens 89277
Bimzelx 160mg solution for injection pre-filled syringe 2 syringes 89278
Tara Kelly

IPU Medicines Information Pharmacist
Highlighted Articles