Home » European Health Data Space
The European Health Data Space Regulation (EHDS) was heralded as a “groundbreaking initiative” by the European Commission when it was initially mooted in 2022. Advocates say it will improve cross-border patient care and deliver a trove of data for research and policymaking. At the end of April, European Parliament approved the agreement to establish the EHDS, edging it closer to realisation. In this article, IPU Editorial Manager Siobhán Kane looks at what the EHDS is, and what is means for patients and healthcare professionals.

The full potential of digital health and facilitating the sharing of health data, have long been discussed. The opportunities generally relate to one of the following: for the individual patient, who has long been frustrated at the lack of progression in digitalisation of their health records, which would make their day-to-day lives easier as all health professionals would not be chained to the availability of whatever paper records are held in their individual office; and for researchers and policymakers, who would be able to harness the power of health data to drive innovation and developments. Effective public health measures need data to drive their effectiveness, and the efficient sharing of data could result in scientific breakthroughs and strategic investments which would herald a new era in how we look at supporting, and funding, the health of our populations.
But there are enormous challenges, and in the Irish landscape they are all too apparent, with failed eHealth strategies that never got off the ground and promises that haven’t materialised. Yet, we seem to be, tentatively, entering a new chapter. At the time of writing, Minister for Health Stephen Donnelly announced that an app allowing patients access to their medical records will be rolled out this year – attendees at the IPU conference in April received a detailed presentation on this very app from the Department of Health’s Head of eHealth and Information Systems, Niall Sinnott. Indeed, Mr Sinnott also described in his presentation how both his Department and the HSE have been building capacity and resources for the last four to five years, to ensure their vision isn’t a pipe dream.
And behind such developments on the domestic front, is an overarching framework at a European level, which will further push change: The European Health Data Space.
MEPs in the European Parliament overwhelmingly approved the inter-institutional agreement on establishing a European Health Data Space (EHDS) on 24 April. It is “a health-specific data sharing framework establishing clear rules, common standards and practices, digital infrastructures and a governance framework for the use of electronic health data by patients and for research, innovation, policy making, patient safety, statistics or regulatory purposes”.
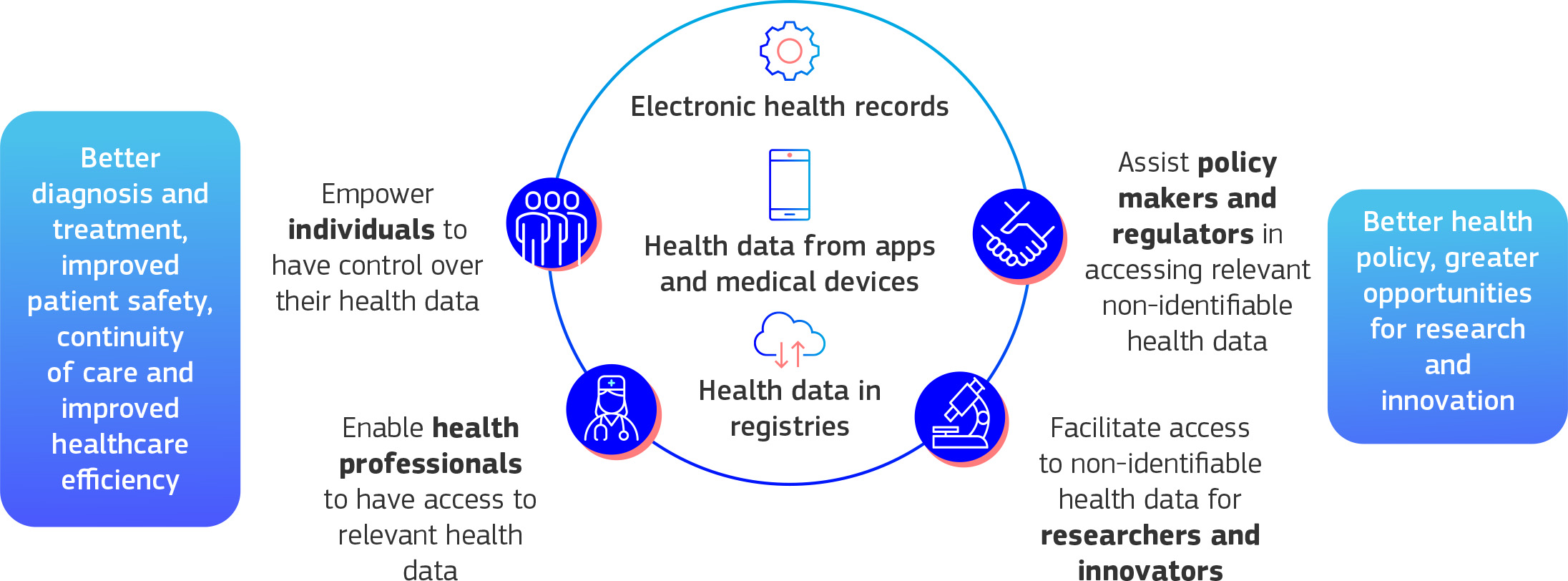
Communications from the European Commission are very clear in terms of the potential benefits of the EHDS. For individuals, they say it will:
For healthcare professionals it will:
And for researchers it will:
Regulators and policymakers will also have easier access to health data for more effective policymaking, while industry will benefit from an EU-wide market for electronic health record systems, with the same standards and specifications.
“Effective public health measures need data to drive their effectiveness.”
Figure 1: European Health Data Space —Objectives
Source: European Commission

A new European Health Data Space Board will be created, co-chaired by a Member State and the Commission, and composed of representatives of the Member States. A stakeholder forum of organisations, researchers, and industry will work with the Board.
Steering groups will also be established that will allow Member States to cooperate at EU level on cross-border digital developments.
While voluntary cooperation among some Member States already exists, the EHDS will turn this into an obligation for all Member States.
The EHDS builds upon the current cooperation for the primary use of data in healthcare within the eHealth Network, which showed its value during the COVID-19 pandemic. Primary use of data relates to the direct use of data collected for the treatment of patients.
Secondary use of health data relates to the re-use of health data originally collected for another purpose by entities such as researchers, policy makers, innovators and industry. The EHDS will establish a framework to regulate this secondary use of data.
One of the main questions being asked about the EHDS, and the question which took up most negotiating hours, is whether patients should have a say when it comes to the reuse of their health data. The negotiators finally reached an agreement on the regulation of the EHDS that provides for EU countries to be allowed to facilitate patients to opt out of the system’s secondary use of their data for studies, except for public interest research.
The EHDS is the first common EU data space to emerge from the European strategy for data. The European Commission published A European strategy for data in February 2020, which focuses on “putting people first in developing technology and defending and promoting European values and rights in the digital world”. It aims to create a single market for data and sets out the path to the creation of Common European Data Spaces. According to the European Commission, Common European Data Spaces will make more data available for access and reuse, and “will help unleash the enormous potential of data-driven innovation”. The Data Strategy proposes nine Common EU Data Spaces, and EHDS is the first to emerge. The others are data spaces for industry and manufacturing, agriculture, finance, mobility, green deal, energy, public administration, skills.

Report: Implementing the European Health Data Space in Ireland
EIT Health is part of the European Institute of Innovation and Technology, which is a body of the European Union. EIT Health has regional centres, including ‘EIT Health Ireland-UK’, which coordinates a network of academic, private and public partners across the health innovation ecosystem (see eithealth.eu > In your region). In November 2023, EIT Health Ireland-UK published the report Implementing the European Health Data Space in Ireland, which examines how ready Ireland is for the EHDS regulation and sets recommendations for policymakers on how to move forward.
The report came about as a result of a pan-European steering committee, coordinated by EIT Health, that was established in early 2023 to assess Member States’ readiness for implementation of the EHDS. Sinéad O’Connor, Adjunct Assistant Professor Emerging Technologies in Healthcare from Trinity College Dublin’s School of Medicine, is the Ireland representative on this steering committee.
The published Report for Ireland includes recommendations, based on stakeholder feedback, including:
Speaking at the Report launch, Elaine Murray, Public Affairs Lead at EIT Health Ireland-UK, said; “The EHDS could be a gamechanger for how healthcare is delivered, and how health research is carried out, in Ireland and across Europe . . . For Ireland to move forward, a significant cultural change is required, transitioning from a focus on data protection to striking a balance between data protection and data sharing to further research that enables improved care for patients. Investment in digital technologies will be required, as well as upskilling the healthcare workforce.”
The Report makes recommendations across six areas, with specific recommendations for EU policymakers and the Irish Government across the following:
When asked by the IPU Review on Ireland’s efforts to prepare for the EHDS, the Implementing the European Health Data Space in Ireland report was cited as further reading. A spokesperson for the Department of Health also described several key actions that are being taken to make sure pharmacies in Ireland will be ready to implement the EHDS:
The Department of Health also cited projects Ireland is involved with, which affect the secondary data uses mentioned in the EDHS: “EHDS will also provide a legal basis for researchers and policymakers to access anonymised or pseudonymised datasets for secondary purposes. Ireland is engaged in several EU-level projects towards implementation:
In December 2023, shortly after the report on implementing the EHDS in Ireland was published, Minister Donnelly announced an investment in digital health infrastructure, as part of the EHDS. This announcement specifically concerned €3.6 million in EU funding to support establishment of a Health Data Access Body (HDAB), which will connect researchers and policymakers with anonymised health datasets. It has a total budget of €6 million (€2.4 million national contribution and €3.6 million in EU funding) and will be delivered in collaboration between the Department of Health, the Health Information and Quality Authority (HIQA) and the Health Research Board (HRB).
Other relevant recent developments include the launch on 21 May of Digital for Care: A Digital Health Framework for Ireland 2024-2030, which “sets out a roadmap to digitally transform health services in Ireland and improve access for patients”. The patient app mentioned earlier in this article, was announced as part of this Framework.
The provisional agreement voted on at the end of April in the European Parliament still needs to be formally approved by the European Council. Once published in the EU’s Official Journal, it will enter into force twenty days later. It will be applied two years after, with certain exceptions, including primary and secondary use of data categories, which will apply four to six years later, depending on the category.
Further updates will be provided in a ongoing manner in the IPU Review. To read more on the EHDS go to health.ec.europa.eu > Improving health systems > eHealth : Digital health and care > EU Health Data Space.
The European Commission provided the following examples of how the EHDS will impact on people.
Example 1: A woman living in Portugal goes on holiday to France. Unfortunately, she falls ill in France and needs to see a local GP. Thanks to the EHDS and MyHealth@EU, a doctor in France will see on their computer the medical history of this patient (facilitated by translation functions). As a result, the doctor can prescribe the necessary medicine based on the medical history of the patient, avoiding for instance products to which the patient is allergic. The prescription information can be shared as well, so it can be used back home in Portugal, or anywhere else in the Union.
Example 2: A health tech company is developing a new AI-based medical decision support tool that assists doctors to make diagnostic and treatment decisions following a review of the patient’s laboratory images. The AI compares the patient’s images with those of many previous patients. Through the EHDS, the company is able to efficiently and securely access a large number of medical images to train the AI algorithm, which in turn optimises its accuracy and effectiveness, before seeking market approval.
Example 3: A man has a medical image of his lungs, taken in the public hospital where he was brought in by the emergency team. Shortly after, he visits his regular doctor in another hospital. Thanks to the EHDS, his doctor can see the medical image performed in the other hospital, thus avoiding a new, unnecessary test.
Siobhán Kane

Editorial Manager, IPU
Highlighted Articles