Home » Autumn Winter Vaccination Programme 2024/25

The Autumn Winter Vaccination Programme began in early October 2024. Similar to previous seasons, co-administration of the seasonal influenza vaccine and COVID-19 vaccine continued to be encouraged for eligible individuals. The COVID-19 booster dose campaign continued until the end of February 2025 while the seasonal influenza (QIV) ended on the 30 April 2025. The administration of nasal flu vaccinations to children aged two to 17 years finished when the LAIV products expired on 24 February 2025. Any children in risk groups requesting an influenza vaccine after this time could be given an injectable vaccine where appropriate.
1,408 community pharmacies offered a seasonal influenza vaccination service during the Autumn Winter Programme. This represented the highest number of pharmacies providing the service since it commenced in 2011.
Vaccines recommended by the World Health Organisation (WHO) are prepared each year, using virus strains similar to those considered most likely to circulate in the forthcoming season. Last year the WHO recommended that B/Yamagata lineages were no longer circulating as they were last detected in March 2020. Therefore, they recommended that the B/Yamagata antigen component of influenza vaccine was no longer required. As a result, the vaccine formulation is moving away from a quadrivalent to a trivalent. The supply of a trivalent live attenuated influenza vaccine was prioritised for the 2024/2025 season. The non-live attenuated vaccine will be adjusted from a quadrivalent to a trivalent formulation for the 2025/2026 season.
There were two seasonal influenza vaccines available free of charge in community pharmacies for eligible individuals through the programme:
Private stock of the Quadrivalent Influenza Vaccine and the Influvac Tetra vaccine were also available through community pharmacy for those persons not eligible for a vaccine under the HSE national programme.
Vaccines supplied for use as part of the HSE national programme were supplied by the National Cold Chain Service with deliveries commencing for Comirnaty JN.1 age 12+ and Quadrivalent Influenza Vaccine (QIV) on 9 September. Supply of Fluenz nasal spray vaccine (LAIV) commenced on 16 September. Deliveries were fortnightly for this influenza season and were aligned with the COVID-19 vaccine delivery schedule.
Recent data released by the Health Protection Surveillance Centre (HPSC) reflects the influenza season 2024/2025 in Ireland to date. The data shows there was a significant increase in the number of laboratory-confirmed influenza cases in comparison to last season. During the 2023/2024 season there were 16,422 laboratory confirmed influenza cases notified while by 14 April 2025 26,666 cases had been reported. As seen within Figure 1, the number of notified cases of laboratory confirmed influenza was highest in late December and early January.
Figure 1: Number of notified cases of laboratory confirmed of influenza by notification week in Ireland between week 40 2019 and week 16 2025.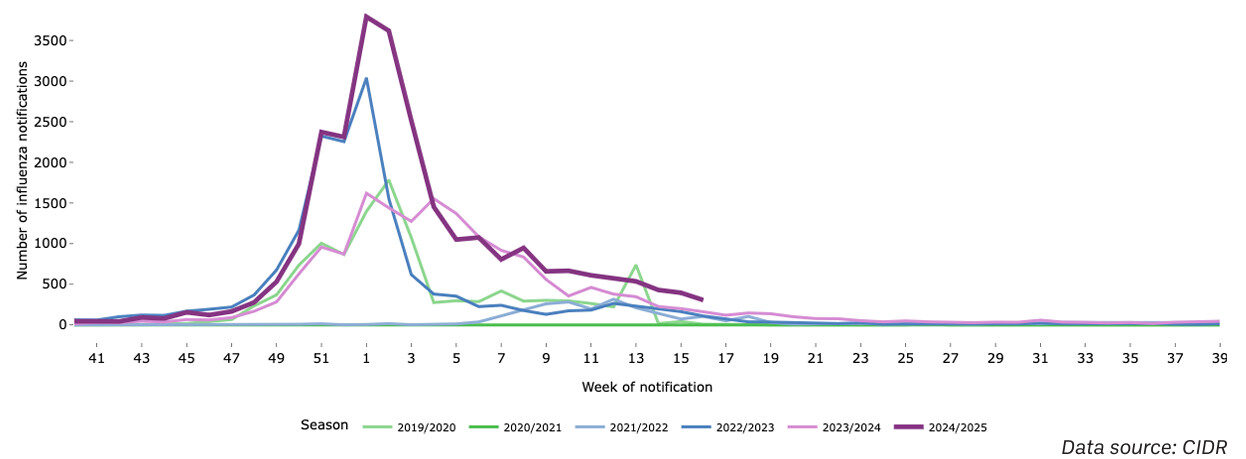
Data source: CIDR
A total of 6,397 of the cases reported during the 2024/25 season were hospital inpatients, including 235 confirmed influenza cases admitted to intensive care units. 317 deaths have been notified. Influenza A was the predominant influenza virus circulating in Ireland. Figure 2 (Age and sex-specific incidence of notified cases of laboratory confirmed COVID-19, influenza and RSV from week 40 2024 to week 16 2025) shows that age-specific rates were highest in those aged 65 years and older and that there was also a relatively high incidence in those aged four years of age and younger.
Figure 2: Age and sex-specific incidence of notified cases of laboratory confirmed COVID-19, influenza and RSV from week 40 2024 to week 16 2025.
Data source: CIDR
“The number of notified cases of laboratory confirmed influenza was highest in late December and early January.”
According to HSE data, over 1,201,000 influenza vaccinations (QIV, LAIV) were administered across all sites (community pharmacies, GPs, hospitals, and other sites) in Ireland during the 2024/25 influenza season. Over 388,000 (32 per cent) influenza vaccinations (QIV, LAIV) were administered by community pharmacists. This showed a significant increase in activity from the 2023/24 season where community pharmacy provided circa 325,600 (28 per cent) of all influenza vaccines.
Vaccine uptake target for children aged 2 to 17 years was set at 50 per cent and for those aged 65 and over 75 per cent. Influenza uptake rates by age group are calculated based on the proportion of individuals in the specified age group who are vaccinated during the period 2 September 2024 to 16 February 2025.
Vaccination uptake information for children aged 2 to 17 years and those aged ≥ 65 years are shown in Figure 3. While a small decrease in vaccination uptake of 74.3 per cent is displayed in those aged ≥ 65 years, an increase to 20.2 per cent from 16.3 per cent last season is observed for children aged 2 to 17 years.
Figure 3: Influenza vaccination uptake, by age group, 2024*-2025 season vs same period 2023-2024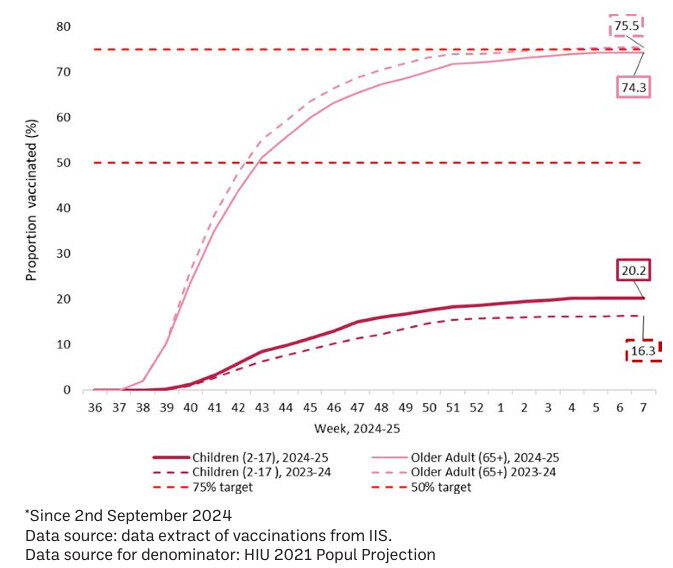
Source: HPSC
Figure 4: Influenza vaccination uptake, by age group, 2024*-2025 season vs same period 2022-2023Figure 4 (shows a further breakdown of vaccination uptake and clearly demonstrates that the vaccine administration rates within the 5 to 12 year age group have experienced a considerable rise which can be attributed to the LAIV Schools Programme.

Source: HPSC
During the 2024/25 Children’s LAIV programme community pharmacy recorded administration rates of circa 127,000 vaccines and held a market share in excess of 57 per cent. This was a significant increase in administration rates on the previous two seasons where community pharmacies recorded more than 78,000 LAIV and held a market share of 45 per cent. Children’s LAIV nasal spray is provided as a traditional service within the retail pharmacy setting and also through the LAIV schools programme.
The rollout of the most recent LAIV schools programme saw some changes to operational aspects. For the 2024/25 season the NIO facilitated community pharmacies and GP practices to link with local primary schools to provide on-site LAIV to children in for all year groups in a school where possible. HSE Teams provided the service to Senior Infants in schools where a primary care contractor had not confirmed they were providing a service.
New functionality to record influenza vaccinations administered at locations outside the retail pharmacy premises was introduced on HSE PharmaVax at the start of the seasonal LAV campaign. This feature enabled community pharmacists to input a temporary location such as a school. Pharmacists can now search for and select the facility ID, name, address, or school roll number in the vaccination details section of HSE Pharmavax, allowing the location and number of vaccinations provided in an offsite location to be identified.
As in previous seasons, the NIO published a Toolkit to Support the Administration of Flu Vaccination to Children in Primary Schools or Community Settings by Primary Care to support the service. The PSI also updated their guidance.
Currently plans are being finalised for the delivery of LAIV during the 2025/26 season.
Over 1.93 million COVID-19 vaccines have been administered by community pharmacy since they were included in the national COVID-19 vaccination programme in 2021. 1,128 community pharmacies offered a COVID-19 vaccination service, and in excess of 175,000 COVID-19 booster doses were administered during the autumn COVID-19 booster campaign. More than 557,000 vaccines were administered during this campaign through all vaccination channels. Community pharmacy displayed a market share of 31.5 per cent which was slightly behind their performance of 35 per cent during the previous autumn booster campaign.
NIAC guidance provided in Immunisation Guidelines Chapter 5a COVID-19 recommended the preferential use of Comirnaty Omicron JN.1 in the Autumn/Winter COVID-19 booster programme.
Recommended groups for the Autumn COVID-19 booster vaccine programme:
– Those aged 60 years and older;
– Those aged 18-59 years living in long term care facilities for older adults;
– Those aged 6 months-59 years with:Permissive vaccination was allowed for those aged 18-59 years and not in one of the above groups who sought vaccination following discussion with their healthcare provider. NIAC guidance recommended an interval following infection or vaccination of six months but allowed for a minimum interval of at least three months since any previous COVID-19 vaccine dose or SARS-CoV-2 infection to be used where there was a need to provide early protection in certain circumstances, for example, planned immunosuppressive therapy or operational reasons. NIAC recommend that the COVID-19 autumn booster vaccine can be co-administered with the influenza vaccine, where a person is eligible for both vaccines, to maximise uptake.
S.I. No. 458 of 2024- Medicinal Products (Prescription and Control of Supply) (Amendment) (No.4) Regulations 2024 was signed into law on 13 September 2024 allowing pharmacists to supply and administer Comirnaty® Omicron JN.1 30mcg/dose dispersion for injection.
This amendment also introduced welcome change to the vaccination record-keeping requirements and consent. The legislation removed the need to record the name, address and telephone number of the patient’s GP to whom the vaccination was administered. In addition, pharmacists were no longer required to notify the patient’s GP within seven days of details pertaining to an influenza vaccine administration.
The legislation allowed for records of the administration to be maintained in electronic form and reduced the time that records are required to be maintained. Vaccination records are now required to be readily available for inspection at the retail pharmacy business for a period of two years from the date of the administration of a vaccine.
The approach to capturing consent for those not able to provide it was brought piece in line with the HSE National Consent policy. A pharmacist now must indicate that the will and preferences of the person were established, and the administration was for the benefit of the person.
These changes brought about a reduction in the administrative burden pharmacists were experiencing and also enabled streamlining of processes.
Community pharmacies continue to play a significant role in the provision of this national programme. The strong delivery of the LAIV schools programme shows yet again the professions ability to embrace new avenues of service provision, increase accessibility to vaccinations and support their communities.
References available on request.
Susan O’Donnell MPSI

Professional Services Pharmacist, IPU
Highlighted Articles