Home » Autumn Winter 2023/24 Vaccination Campaign

The Autumn Winter Vaccination Campaign saw the seasonal influenza vaccination and the autumn COVID-19 booster dose programmes offered at the same time to recommended cohorts. The 2023/24 campaign began in early October. In line with National Immunisation Advisory Committee (NIAC) advice, co-administration of the seasonal influenza vaccines and COVID-19 vaccines was encouraged for eligible individuals. The COVID-19 booster dose programme continued until the end of February 2024 while the seasonal influenza programme ended on the 30 April 2024.
1,344 community pharmacies offered a seasonal influenza service during the Autumn Winter Campaign.
The recommended groups for the 2023/24 season who were eligible to receive the free influenza vaccine through the HSE national programme were those who were:
Initially only children aged 2-12 years were recommended to receive LAIV nasal spray, however this recommendation was extended in January 2024 to allow for children aged 13-17 years to also receive this vaccine free of charge.
There were two seasonal influenza vaccines available free of charge in community pharmacies for eligible individuals through the programme:
Private stock of the Quadrivalent Influenza Vaccine and the Influvac Tetra vaccine were also available through community pharmacy for those persons not eligible for a free vaccine under the HSE national programme.
Vaccines supplied for use as part of the HSE national programme were supplied by the National Cold Chain Service with deliveries commencing the week beginning 18 September 2023. Deliveries were fortnightly for this influenza season and were aligned with the COVID-19 vaccine delivery schedule.
In Ireland during the 2023/24 influenza season 16,422 laboratory-confirmed influenza cases were notified to the Health Protection Surveillance Centre (HPSC) by 7 May 2024.
A total of 4,075 of the cases reported during the 2023/24 season were reported as hospital inpatients, including 118 confirmed influenza cases admitted to intensive care units. Age-specific rates were highest in those aged 65 years and older. For the season to date, 214 deaths were notified in influenza cases.
Figure 1 shows that Influenza A was the predominant influenza virus circulating in Ireland during the 2023/24 season with 14,756 cases notified to the HPSC. 1,672 Influenza B and 13 influenza coinfections cases were also notified.
Figure 1: Number of laboratory confirmed influenza notifications by influenza type/subtype and week for the 2023/2024 Season.
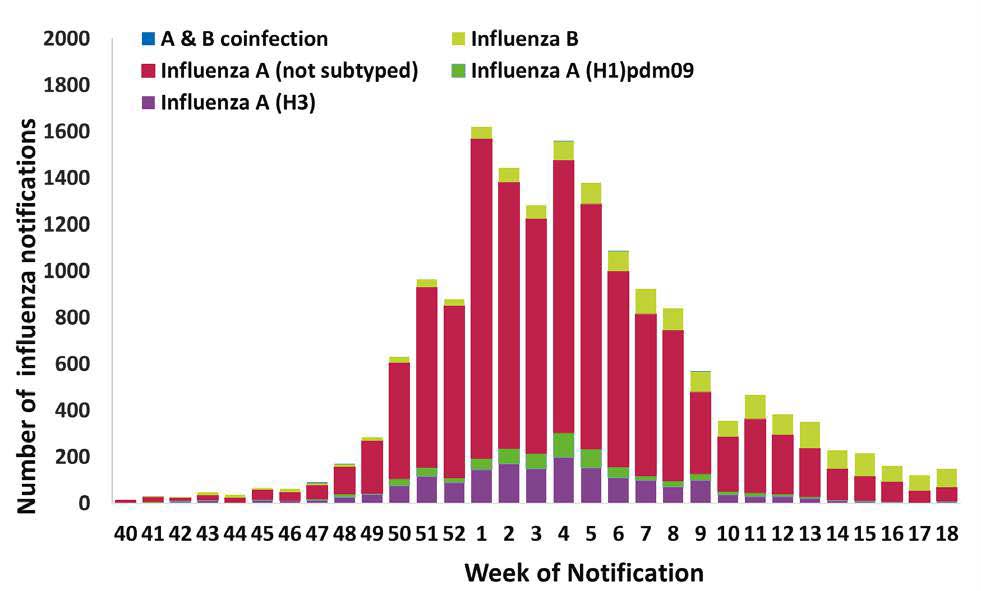
Source: HPSC
According to HSE PharmaVax data a total of 1,175,672 influenza vaccinations (QIV, LAIV) were administered across all sites (Community Pharmacies, G.P.s, Hospitals, and other sites) in Ireland during the 2023/24 influenza season with 325,661 (27.7 per cent) influenza vaccinations (QIV, LAIV) being administered by community pharmacists.
Vaccination uptake information for the Irish population is available for two cohorts- namely those aged 2 to 17 years and those aged 65 years and older. Figure 2 shows the uptake rates across these cohorts for both the 2022/23 and the 2023/24 seasons. The 2023/24 influenza season saw 16.22 per cent of the population aged 2 to 17 years vaccinated and 75.5 per cent of those aged ≥65 years.
Figure 2: Influenza vaccination uptake, by age group, 2023*-2024 season vs same period 2022-2023
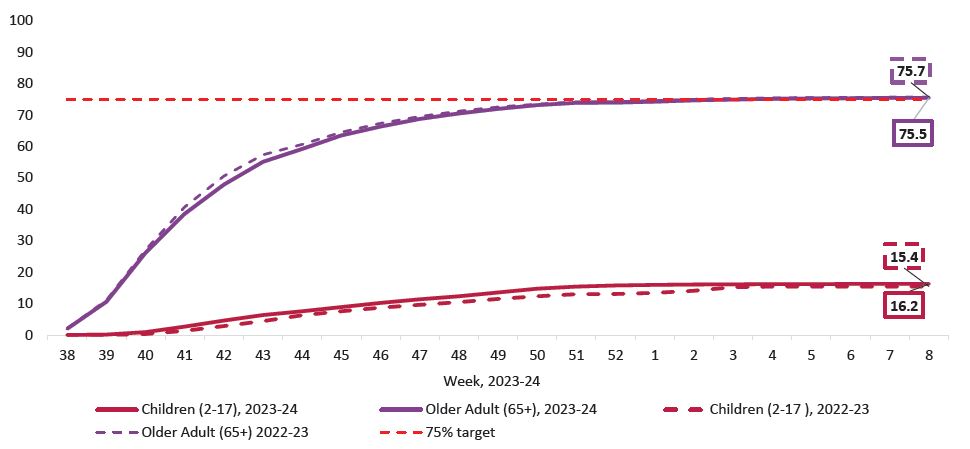
Source: HPSC
The LAIV nasal spray for children was introduced in Ireland for the first-time in 2020/21. A high uptake of LAIV nasal spray is required in eligible children to achieve the population level benefits of the programme. The uptake to date overall for children aged 2-17 has remained below 20 per cent. A key Public Health priority was to improve the uptake of flu vaccine in children aged 2-12, and to achieve this a number of initiatives were planned, including the delivery of LAIV nasal spray in Primary School settings. The delivery of primary school children’s flu vaccination in a school setting in the UK has seen uptake levels improve considerably. Uptake rates in 2022/23 were 56 per cent.
HSE mobile Vaccination teams provided children’s LAIV nasal spray on-site in schools between October & December 2023 to Senior Infants in primary schools and eligible children in primary age special schools.
The NIO facilitated community pharmacies and GP practices to link with local primary schools to provide on-site LAIV to children in other year groups where possible. The NIO published a ‘Toolkit to Support the Administration of Flu Vaccination to Children in Primary Schools or Community Settings by Primary Care’ to support the service. The PSI updated their ‘Guidance to support pharmacies in providing safe vaccination services offsite from the pharmacy premises’ to provide clarity around certain aspects of this service.
Community pharmacies recorded almost 78,000 LAIV administrations and a market share of 45 per cent of the total LAIV nasal spray activity for the 2023/24 season. These figures mirrored that achieved in the 2022/23 season. During the 2021/22 season the market share was 40 per cent.
This strong performance has cemented the valuable role that community pharmacy plays in the delivery of this service. Currently plans are being formalised for the 2024/25 influenza season. The HSE recently issued a communication encouraging community pharmacies who wish to engage in the LAIV Schools Programme to reach out to mainstream primary schools to explore if they would be interested in offering LAIV nasal spray on-site.
There are four types of influenza viruses, type A,B, C and D. Influenza A and B are the main viruses of focus for seasonal influenza.
The use of QIVs has replaced the use of trivalent vaccines (TIVs) in Europe for seasonal influenza vaccination. TIVs comprise of two influenza A antigens and one influenza B antigen (either the B/Victoria or B/Yamagata) depending on which was expected to contribute most to annual influenza burden in the next season, and QIVs comprise of two influenza A antigens along with antigens of both the B/Victoria and B/Yamagata lineages.
In March 2024, the European Medicines Agency (EMA) Emergency Task Force (ETF) issued a recommendation regarding the replacement of QIVs with TIVs in the EU. This recommendation was made as there had been no confirmed detection of the naturally occurring B/Yamagata virus since March 2020. They have recommended that, ideally, the antigens of the B/Yamagata lineage should be removed from LAIVs for the 2024/25 influenza season as this could pose a risk of the lineage being reintroduced into humans. For inactivated influenza vaccines such as QIV and TIV they have recommended that the B/Yamagata lineage should be removed for the 2025/26 season as there is no public health concern requiring an immediate transition, and vaccine availability is of primary importance. Continuous monitoring to confirm the disappearance of B/Yamagata will be undertaken.
The WHO recommends that trivalent vaccines for use in the 2024/25 northern hemisphere influenza season contain the following:
Egg-based vaccines:
Cell culture- or recombinant-based vaccines:
For quadrivalent egg- or cell culture-based or recombinant vaccines for use in the 2024/25 northern hemisphere influenza season the B/Yamagata lineage component remains unchanged:
Egg-, cell- or recombinant-based vaccines
No confirmation of the vaccines that will be used in the upcoming influenza season is available as of yet. The IPU Flu Hub will be updated as information becomes available for the season ahead.
Over 1.7 million COVID-19 vaccines have been administered by community pharmacy since they were included in the national COVID-19 vaccination programme in 2021. 998 community pharmacies offered a COVID-19 vaccination service, and 269,683 COVID-19 booster doses were administered during the autumn COVID-19 booster programme. 770,522 vaccines were administered during this campaign through all vaccination channels and community pharmacy maintained a market share of 35 per cent.
On August 30 2023 the European Medicine Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended authorising an adapted Comirnaty vaccine targeting the Omicron XBB.1.5 subvariant. The National Immunisation Advisory Committee (NIAC) recommend the preferential use of the newly adapted monovalent vaccine, Comirnaty Omicron XBB.1.5 in the Autumn COVID-19 booster programme. S.I. No. 451 of 2023. Medicinal Products (Prescription and Control of Supply) (Amendment) (No.6) Regulations 2023 was signed into law allowing pharmacists to supply and administer Comirnaty Omicron XBB.1.5 30mcg.
NIAC recommended an autumn COVID-19 booster vaccine for:
Those aged 18-49 years and not in one of the above groups who sought vaccination following discussion with their healthcare provider could also receive an autumn COVID-19 booster dose. NIAC guidance allowed for a minimum interval of at least three months since any previous COVID-19 vaccine dose or SARS-CoV-2 infection to be used where there was a need to provide early protection, or to facilitate co-administration with influenza vaccine.
”Over 1.7 million COVID-19 vaccines have been administered by community pharmacy since they were included in the national COVID-19 vaccination programme in 2021.”
The strong performance of community pharmacy teams in delivering these two key national vaccination campaigns shows the commitment of the profession to expand their role, promote the national vaccination programmes and support their local populations.
Susan O’Donnell MPSI

Professional Services Pharmacist, IPU
Highlighted Articles