Home » FMD in Ireland – one year from the end of ‘use and learn’
The ‘use and learn’ period for the Falsified Medicines Directive (FMD) ended in Ireland on 30 May. In this article, Colin Mac Namee, Operations Manager with the Irish Medicines Verification Organisation (IMVO), provides an overview of FMD in pharmacies and the resources and supports available from the IMVO.

The Falsified Medicines Directive (FMD) was adopted by the European Union in 2011 to prevent falsified medicines entering the legal supply chain and strengthen and harmonise patient protection across Europe.
The Irish Medicines Verification Organisation (IMVO) is responsible for setting up and managing the Irish Medicines Verification System (IMVS) which is part of the European Medicines Verification System. We support pharmacies, hospitals and wholesalers (end-users) to connect to the IMVS, assist with any problems that arise, and work with end-users and marketing authorisation holders (MAHs) to minimise avoidable alerts.
The ‘use and learn’ period for FMD in Ireland ended on 30 May 2022. Since then, the alert rate in community pharmacies and hospitals has significantly reduced while scanning rates have increased, and greater FMD awareness amongst end-users connected to the IMVS is apparent. MAHs have continued working on alert reduction and managing alerts that may occur due to data upload issues. To mark one year from the end of ‘use and learn’, IMVO has provided updated guidance on reducing alerts in community pharmacies and hospitals based on learnings from the last twelve months.
The Safety Features Oversight Group (comprising IMVO, Department of Health, PSI, HPRA, HSE and Private Hospitals Association), continues to oversee progress with FMD implementation in Ireland. IMVO’s role within this group is to report on progress with FMD in Ireland and the rest of Europe, and provide input on behalf of IMVO’s members, the IPU, Irish Pharmaceutical Healthcare Association, Medicines for Ireland, Pharmaceutical Distributors Association, and the Association of Pharmaceutical Parallel Distributors Ireland. The Safety Features Oversight Group approved the transition out of ‘use and learn’ in May 2022. Since then, all alerts must be investigated, and a pack should not be supplied to the patient until the root cause of the alert has been identified and falsification has been ruled out.
Alerts
Across Europe the average weekly alert rate is 0.17% while in Ireland the average weekly end-user alert rate has settled at approximately 0.05%, meaning that one alert is generated for every 2000 packs that are scanned in Ireland. Huge progress has been made in reducing and avoiding alerts in pharmacies and hospitals over the last twelve months. During April 2023, 60% of pharmacies in Ireland did not generate an alert and a further 20% generated no more than one alert during the month.
IMVO works continuously with pharmacy teams assisting with FMD related queries, and providing guidance to reduce avoidable alerts such as double dispensing. Software and scanner issues have been largely eliminated through close cooperation between IMVO and pharmacies and the providers of FMD software. IMVO monitors the patterns of alerts generated and immediately requests MAHs to resolve data issues identified as causing alerts in pharmacies. In our experience, MAHs act very quickly to correct these issues.
In recent weeks the largest driver of avoidable alerts in community pharmacies has been ‘packs decommissioned at multiple locations’ when the alert is generated because the pack has previously been decommissioned in another location. Our analysis shows that many of these alerts are because of decommissioned packs being borrowed from other pharmacies or hospitals. The IMVO team has developed a suite of resources, discussed later in this article, to help avoid these alerts.
IMVO continues to actively engage with and assist pharmacies with alerts. Our outreach has expanded this year to include lectures on FMD and alert management to pharmacy and pharmacy technician students in University College Cork, Technological University Dublin and Technological University of the Shannon – Athlone Campus.
Scanning
There are approximately 200 million FMD transactions across Europe every week, more than 10 billion in total during 2022. In Ireland there are currently over 1.7 million transactions every week. However, Ireland continues to have a relatively low rate of decommissioning compared with the rest of Europe according to the monitoring reports published every two months by the European Medicines Verification Organisation (EMVO).
We have noticed that the incorrect mode or tab can be inadvertently selected on certain FMD software systems during dispensing, resulting in packs being scanned in ‘verify’ mode instead of ‘dispense’ or ‘supply’ mode — this means that the scan is not recorded as a decommissioning scan. Dispensary teams should check that the FMD software is in the correct mode when decommissioning packs as dispensed or supplied. Similarly, it is important to ensure that the ‘destroy’ or ‘sample’ modes are only selected when either of these actions is intended as several alerts are generated in pharmacies every week due to either of these two modes being selected in error.
As an additional check, some community pharmacies conduct verification scans when packs are delivered to them. If this is done in your pharmacy, it is important to ensure that the ‘verify’ mode/tab is selected in the FMD software for these scans, or else the packs could be decommissioned in error which will cause future alerts when they are decommissioned as dispensed/supplied.
Ireland continues to have a relatively low rate of decommissioning compared with the rest of Europe according to the monitoring reports published every two months by the European Medicines Verification Organisation (EMVO).
Since the end of ‘use and learn’ the IMVO team has continued to develop resources to assist dispensary teams. The FAQs on imvo.ie are regularly updated and our newsletter, FMD In Focus, contains useful tips and advice on reducing alerts. During June, we will circulate copies of the FMD reference cards shown here to hospital and community pharmacies throughout Ireland. The cards are designed as a handy reference guide for dispensary teams based on the most common avoidable alerts in pharmacies.
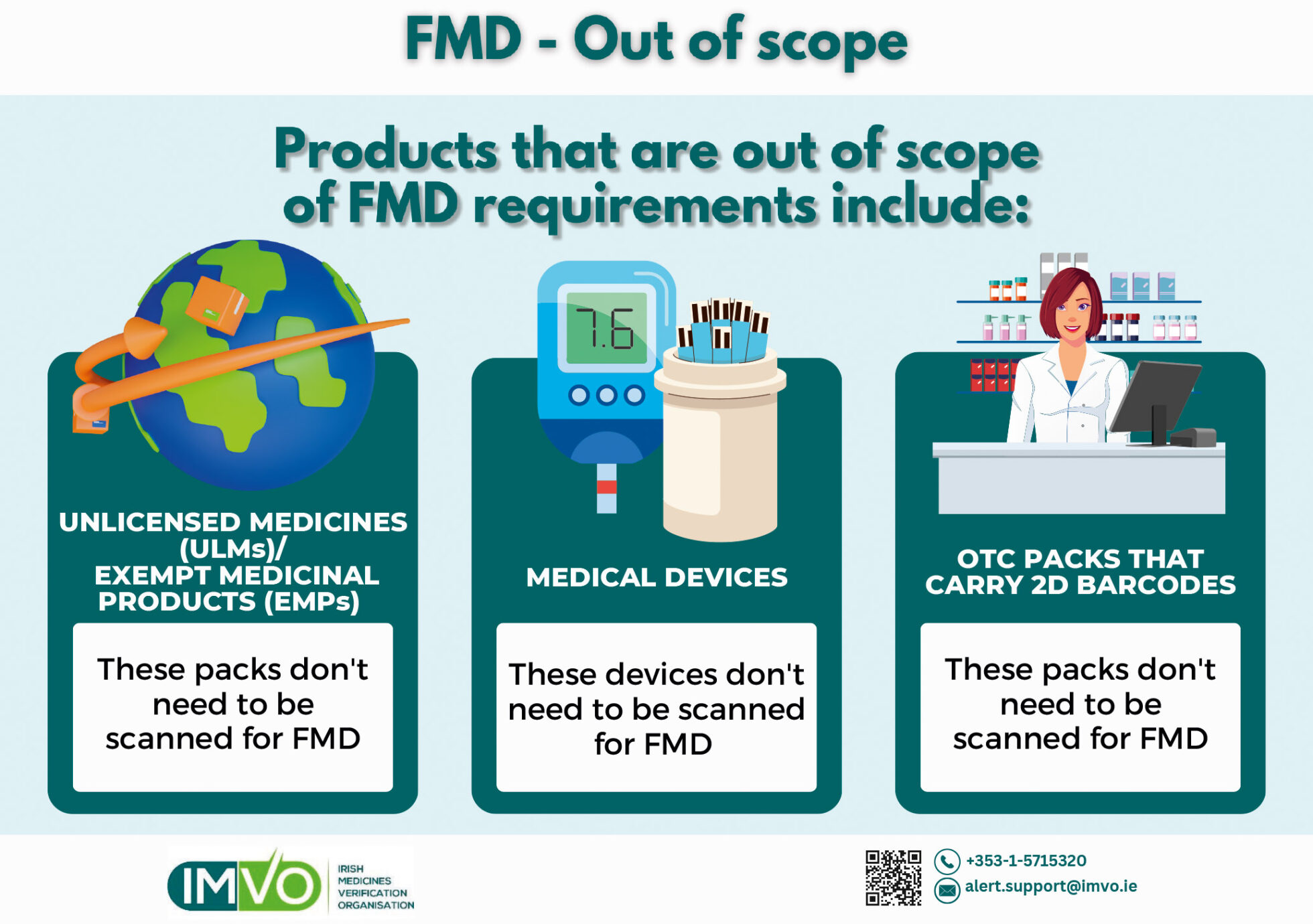
Not all products that carry 2D barcodes are in scope of FMD, the requirement for unique identifiers and anti-tampering devices applies mainly to prescription-only medicines — see Figure 1.
Unlicensed Medicines (ULMs)
As products authorised in other markets but not in Ireland, ULMs are out of scope of FMD in this country, and should not be scanned. If you do scan a ULM by mistake and get an alert, you may supply the pack unless you have overriding concerns that a falsified medicine is involved, or believe the pack has been interfered with or the pack is flagged as expired, recalled, withdrawn, stolen or destroyed. Always check the anti-tampering device (if there is one). This guidance was drawn up in consultation with the HPRA.
Medical devices/OTC packs
Many medical devices and OTC packs now carry 2D barcodes. These devices or packs should not be scanned for FMD, as doing so will generate an exception message in your FMD software.
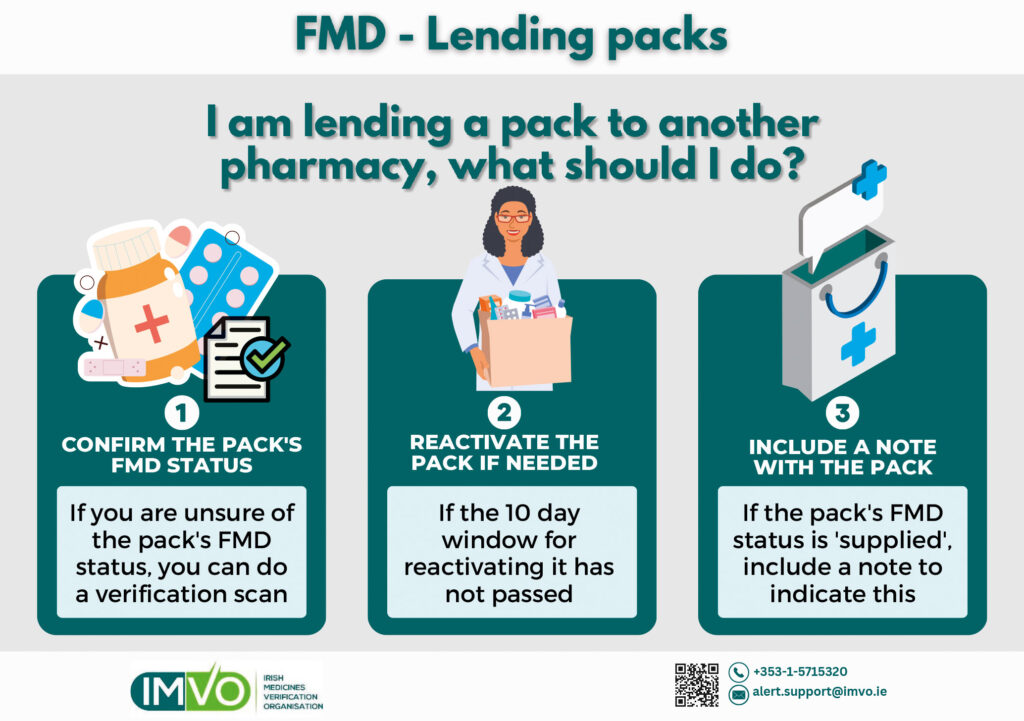
Lending
If you are lending a pack to another pharmacy (or hospital), and are unsure of the pack’s FMD status, you should do a verification scan to check if the pack is ‘active’ — see Figure 2.
If the pack has been decommissioned less than ten days ago, you can reactivate the pack before you lend it to another pharmacy.
If the pack is decommissioned and you cannot reactivate it, you should include a note to that effect with the pack, so the receiving pharmacy knows it has been decommissioned and not to scan the pack again.
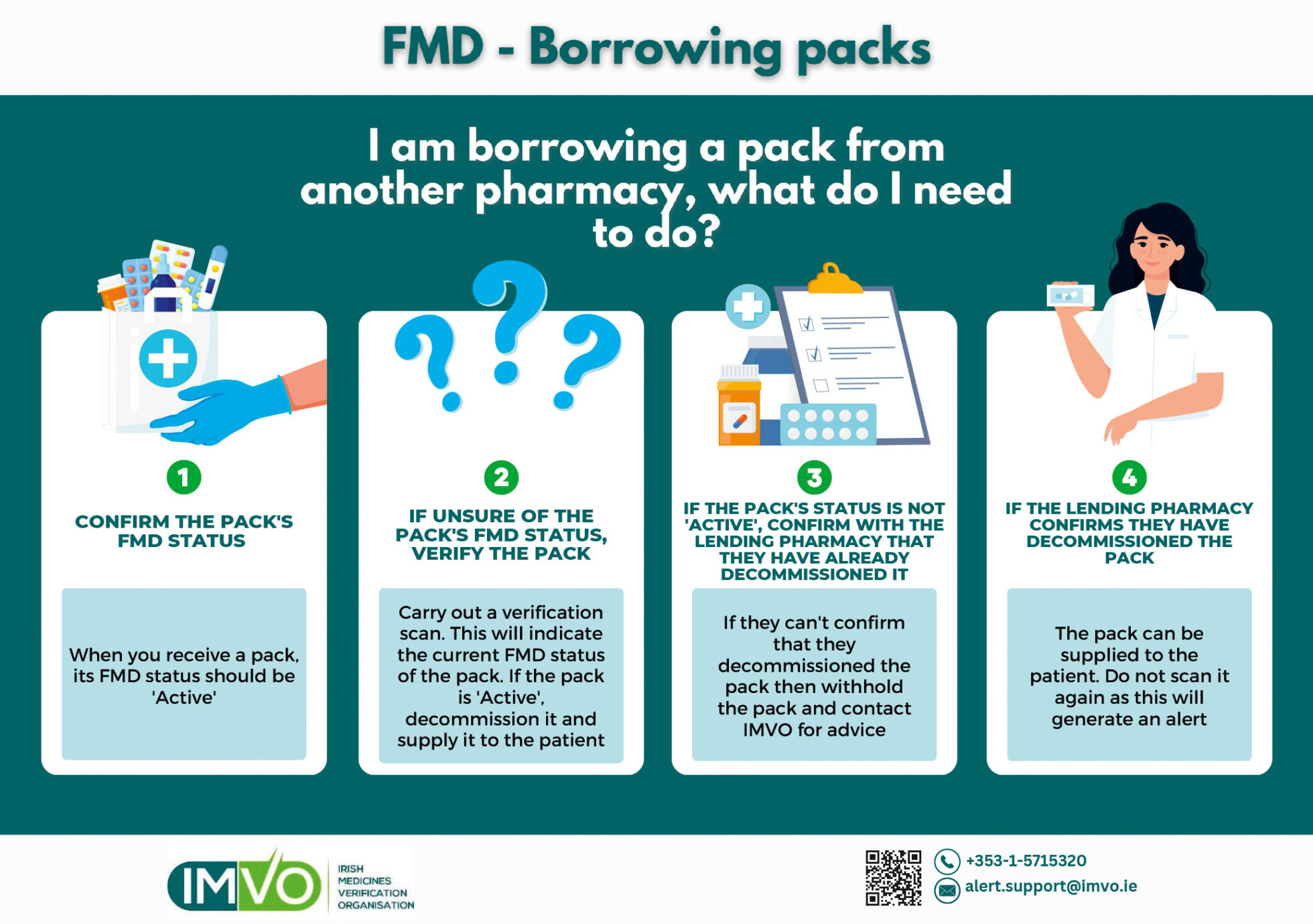
Borrowing
If you are borrowing a pack from another pharmacy or hospital you should check the pack’s FMD status with the lender — see Figure 3. Note that packs borrowed from a hospital will likely be decommissioned, as hospitals are legally permitted to decommission packs at goods inwards (and generally do this) and may be beyond the ten-day window for recommissioning the pack when it is being loaned.

A verification scan will show the pack’s FMD status and if required, the borrowing pharmacy can confirm that the pack was decommissioned by the lender prior to receipt; these packs do not need to be scanned again and the pack can be supplied to the patient. If the pack was not decommissioned by the lender, contact IMVO for assistance.
Destroying packs
When decommissioning packs to ‘destroy’ them, it is important to check a few things — see Figure 4:
NMVS Alerts is the name of the alert management system available to assist end-users, IMVO and MAHs to easily manage alerts, communicate with each other and record the root cause of an alert. It is a web-based platform and is free of charge for pharmacies. Some of the features of value to pharmacies are outlined below:
Detailed information on FMD, recordings of our webinars for pharmacies, and a comprehensive set of FAQs is available on imvo.ie. The live status of the IMVS can also be checked via our website or directly at status.nmvo.eu
Our service desk is open during office hours and in the evenings and on weekends/public holidays to support end-users:
To contact us about an alert, use NMVS Alerts or email any alert queries to alert.support@imvo.ie or call 01 571 5320.
The IMVO operations team is available to provide one-to-one support sessions via phone, Zoom or Teams to pharmacists and/or their dispensary teams — these can be arranged through the contact details above.
Colin Mac Namee

Operations Manager, Irish Medicines Verification Organisation
Highlighted Articles