Home » Medication Without Harm
Medication is the most common therapeutic intervention and our work as pharmacists, pharmaceutical assistants and technicians, aims to ensure safe, effective and efficient use of medicines. The IPU Review will carry a series of articles on medication safety over the coming months, to provide regular practice focused updates to aid you in ensuring medication and patient safety in your practice.
The aim of this series is to provide updates on issues such as:
In the first article in this series, Ciara Kirke, medication safety lead with the HSE, provides an overview of the WHO Global Patient Safety challenge, ‘Medication Without Harm’.
Reducing medication-related harm and improving medication safety is the focus of the current WHO Global Patient Safety Challenge, ‘Medication Without Harm’. This is the third challenge, following on from the very successful ‘Clean Care is Safer Care’, and the ‘Safe Surgery Saves Lives’ challenges.
Why?
Medication is a leading cause of avoidable harm in healthcare systems across the world. Irish research tells us:
At least half of this medication-related harm is potentially preventable.
The challenge goal is to reduce serious avoidable medication-related harm by 50 per cent over five years. The WHO framework identifies three key areas for priority action and four domains in which actions are needed.
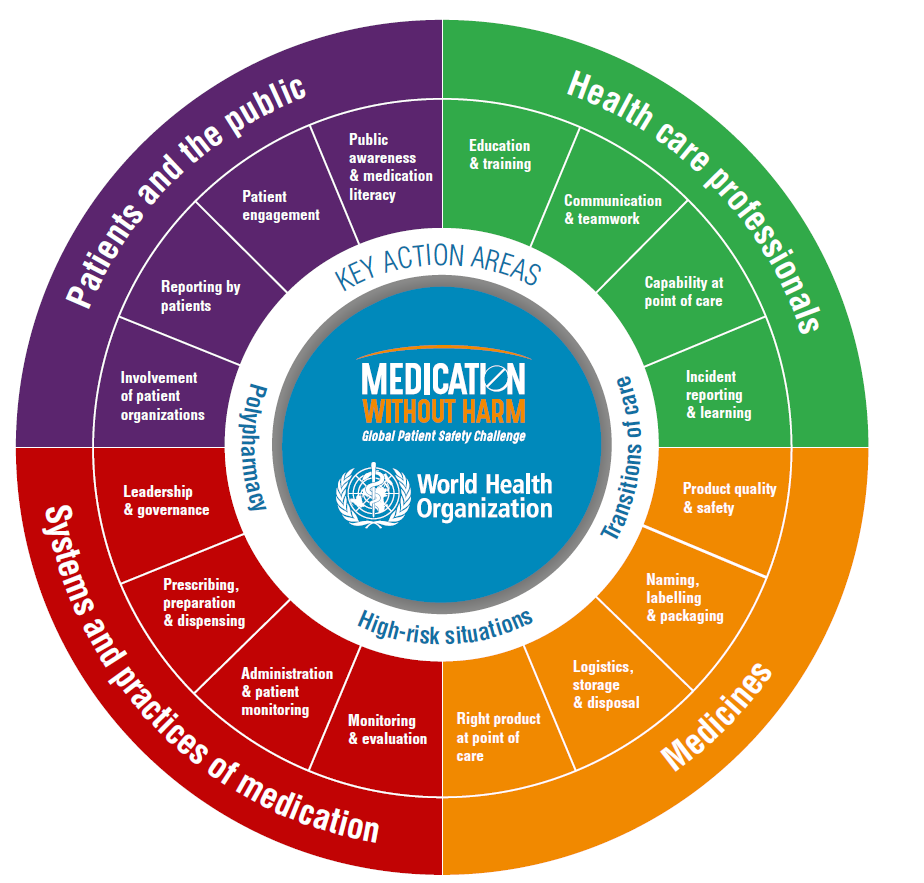
Figure 1: The Strategic Framework of the Global Patient Safety Challenge
This image is available at strategic-framework-medication-without-harm86c06fafdf0b4294bd23ec9667dfb95d.pdf (who.int)
Three key priority areas for urgent action identified by the WHO target the most at-risk processes and patient groups:
| A | Antimicrobials |
| P | Parenteral potassium and electrolytes |
| I | Insulins |
| N | Narcotics/opioids |
| C | Chemotherapeutic agents |
| H | Heparins and other anticoagulants and antiplatelet medicines |
It should also be noted that anti-inflammatory drugs (NSAIDs) and diuretics are also associated with a substantial burden of harm; and
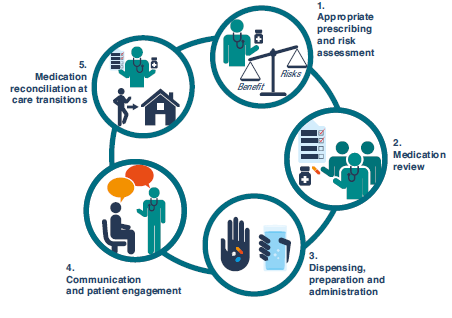
The pharmacy team is in a powerful position to think globally and act locally, supporting patients and staff to reduce potential medication-related harm. The pharmacy team can promote patient safety at each of the key steps highlighted by the WHO:
Figure 2: Key Steps for Ensuring Medication Safety (available p. 8 at
https://www.who.int/docs/default-source/patient-safety/who-uhc-sds-2019-11-eng.pdf)
The World Health Organisation (WHO) has outlined on many occasions that medication safety is a focus area for improvement. To support community pharmacists in this area, the IPU has launched the Medication Safety Section in the IPU Review. Here are some of the concepts that will be explored:
Adverse event: An adverse event is an untoward medical occurrence after the exposure to a medicine, which is not necessarily caused by that medicine.
Adverse reaction: An adverse reaction is a response to a medicinal product which is noxious and unintended. This includes any harm following an overdose, misuse or error regarding that medicinal product.
High-risk medicines: High-risk medicines are those medicinal products that have a higher risk of causing significant patient harm or death when used in error. Some hospitals in Ireland use the acronym “A-PINCH” to raise awareness about high-risk medicines. This acronym stands for Anti-infective agents, Potassium, Insulin, Narcotics and sedatives, Chemotherapy, and Heparin and other anticoagulants.
LASA — Look Alike, Sound Alike, medicines: There are many look-alike or sound-alike drugs that could result in medication errors. These groups of medicines are commonly referred to as LASA or SALAD. According to the World Health Organisation (WHO), medication errors and unsafe medication practices are leading causes of patient harm globally. LASA medicines are a well-recognised cause of medication errors that are due to orthographic (look-alike) and phonetic (sound-alike) similarities between medicines, which can be confusing.
Polypharmacy: Even though there is no set definition for this concept, polypharmacy is interpreted as the concurrent use of five or more medications, including over-the-counter medicines, prescription medicines and/or traditional and complementary medicines.
Transition of care: Transition of care are the various points where a patient moves to, or returns from, a particular physical location, or makes contact with a healthcare professional for the purposes of receiving health care. Therefore, during the patient journey there is a flow from primary care settings to secondary care settings and vice-versa. Throughout the transition of care, there is a high probability that the patient’s medication (if there was any), may change between the different stages of the transition of care.
There have been sporadic reports from hospitals of patients mixing up cyclizine and dexamethasone tablets. Patients on regular chemotherapy often receive a prescription for cyclizine tablets (on an ‘as required’ basis), at the beginning of their treatment for nausea and vomiting. Certain chemotherapy regimens require patients to take a high dose of dexamethasone tablets during each cycle. In some cases, patients have mistakenly taken all of their cyclizine tablets instead of dexamethasone. In many of these cases, the patients have been admitted to hospital for management of side-effects and observation.
A common theme running through these incidents is that the patients were already several cycles into their treatment, leading to the conclusion that complacency is a factor in the mix-ups. It is also highly likely that the similar appearance of both products contributes to the problem. Generally, cyclizine tablets and dexamethasone tablets are dispensed in brown tablet vials and the tablets themselves are broadly similar in appearance.
Suggested ways to reduce the risk of patients selecting the wrong medicine are:
It can be useful to have contact details for your local oncology department or oncology pharmacist, so you can flag this issue to them, should it occur in your practice.
Ciara Kirke

Clinical Lead, HSE National Medication Safety Programme
Highlighted Articles