Learning outcomes
At the end of this article, you will be able to:
- Outline key changes in GINA 2025 Guidelines, which relate to community pharmacists in Ireland;
- Compare GINA asthma management Track 1 and 2;
- Describe all considerations in the GINA ‘Assess, Adjust, Review’ personalised treatment of asthma; and
- Revise your current practice(s) to ensure they align with the updated GINA guidance.
Next month: A follow-up article in the November
IPU Review will explore the value of asthma action plans and highlight practical tools and resources for supporting patients.
The Global Initiative for Asthma (GINA) works with healthcare professionals, patient representatives, and public health officials around the world to reduce asthma prevalence, morbidity, and mortality.
This article provides a summary of the 2025 update to the GINA Global Strategy for Asthma Management and Prevention, with a focus on key developments relevant to community pharmacy practice in Ireland. A follow-up piece next month will explore the value of asthma action plans and highlight practical tools and resources for supporting patients.
GINA 2019 — a shift in asthma management
In 2019, the GINA Global Strategy published new recommendations, which were considered a paradigm shift in asthma management. The main change was that SABA-only treatment (short-acting beta-agonists) is no longer recommended for the treatment of asthma in adults and adolescents. This change was based on strong evidence that SABA-only treatment increases the risk of severe exacerbations and asthma-related death, and that adding any ICS (inhaled corticosteroid) significantly reduces the risk.
Before 2019, it was recommended to start patients on a SABA inhaler, such as Ventolin or Bricanyl, to be used as required. A low dose ICS, such as Becotide and Flixotide, was recommended for patients using their SABA inhaler three times a week or more, or who were symptomatic three times a week or more, or who woke at night due to asthma symptoms at least once a week.
In 2019, the GINA update stated that “there is strong evidence that SABA-only treatment does not protect patients from severe exacerbations, and that regular or frequent use of a SABA increases the risk of a serious exacerbation”.
The recommendation in 2019 included at Step 1, for mild asthma, as-needed low-dose ICS-formoterol, such as Symbicort, or a low dose ICS taken whenever SABA is taken. Daily low dose ICS is no longer recommended at Step 1. The reason for this change is that patients with symptoms less than twice a month were considered unlikely to use their ICS regularly, leaving them exposed to the risks of SABA-only treatment.
GINA’s updates from 2019 to 2025 reflect a steady evolution in asthma care, building on the seismic shift of 2019 and moving toward more personalised, preventive, and environmentally aware strategies.
GINA 2025: Key changes
- Age-specific recommendations: Provides targeted recommendations for diagnosing and managing asthma in patients across different age groups, including guidance on patients under five years;
- Biomarker integration: Encourages use of biomarkers, including blood eosinophils and FeNO to identify Type-2 inflammation and tailor treatments more precisely for individual patients;
- Clarifies Track 1 and Track 2 treatment plans, which were introduced in 2020-21, and emphasises the risks associated with SABA overuse;
- Patient engagement and individualised treatment: GINA 2025 continues to emphasise the importance of shared decision-making between patients and healthcare professionals;
- Monitoring and adherence: The report recommends routine assessment of symptom control, risk factors, adherence, and inhaler technique; and
- Climate change impact: The impact of extreme weather and climate change on asthma is acknowledged, noting factors such as increased pollen counts, air pollution, and extreme temperatures.
GINA 2025 Adults and Adolescents — Track 1 and Track 2
As mentioned above, 2019 introduced a fundamental change in asthma management.
Research showed that patients with apparently mild asthma are still at risk of serious exacerbations, including fatalities. Regular use of SABA inhalers is also associated with increased airway responsiveness, reduced bronchodilator effect, increased allergic response, and increased eosinophils, thereby causing a vicious cycle of overuse. Starting patients with a SABA inhaler trains them to see it as their primary asthma treatment, and poor adherence with ICS is almost inevitable. Furthermore, four to five courses of oral corticosteroids over a lifetime increase the risk of adverse events such as osteoporosis, diabetes, cataracts, and heart failure.
While low-dose ICS was shown to be very effective, adherence was shown to be very low. Many studies showed that adherence in the community setting was only 25-50 percent of the prescribed dose, and moreover, most patients with symptoms less than twice a week do not want to take medication every day.
Image 1: GINA 2025 — Track 1 and Track 2 approach
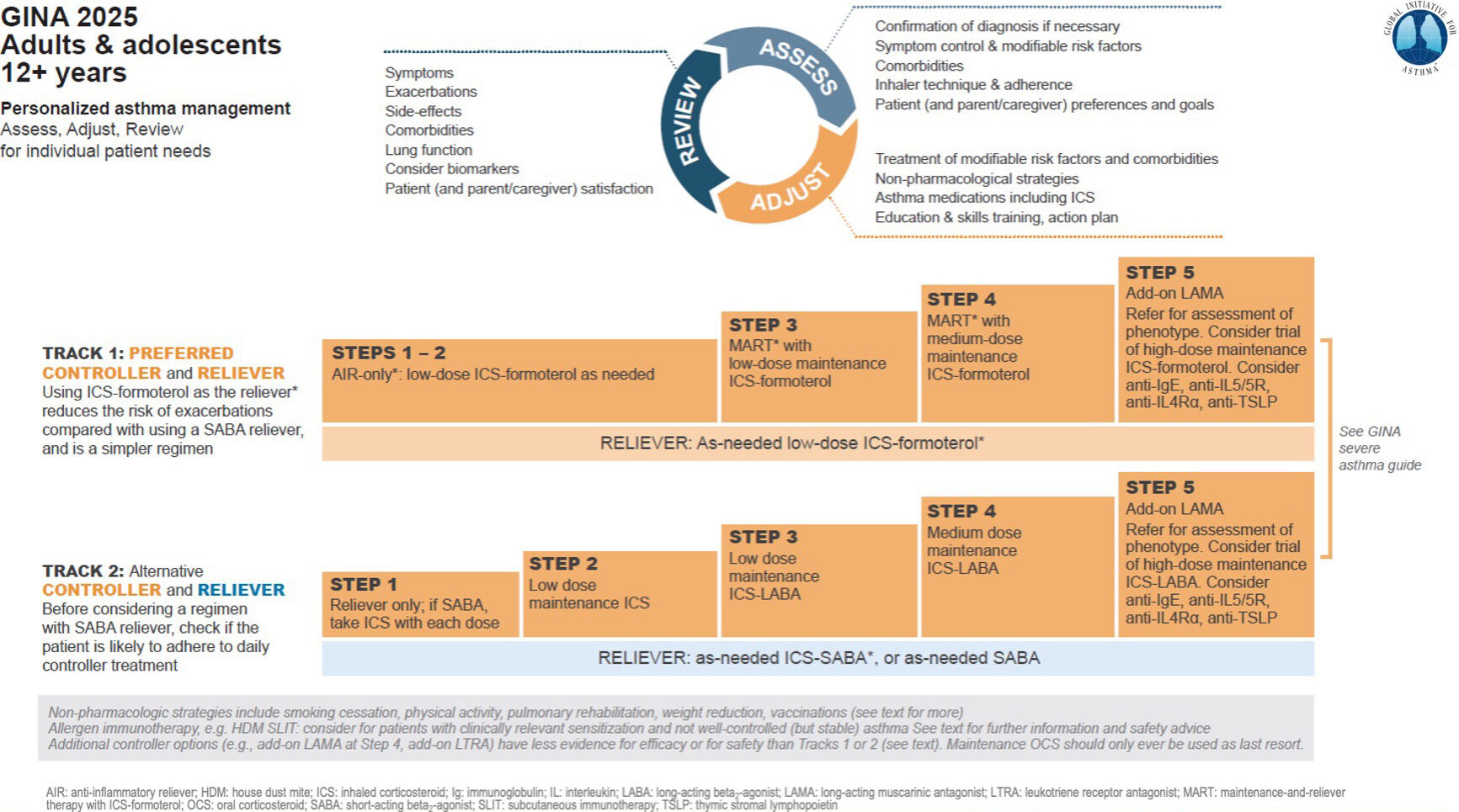
The 2025 update has kept the Track 1 and Track 2 approach introduced in 2020-21, and builds upon the changes made in 2019.
In Track 1, the reliever inhaler is a low-dose ICS-formoterol inhaler, such as Symbicort 100/6, or Bufomix 80/4.5). In Steps 1-2, this is used as needed. At Step 3, maintenance and reliever therapy (MART) is introduced, but again using low-dose ICS-formoterol. At Step 4, MART is maintained with a medium dose maintenance ICS-formoterol, such as Symbicort 200/6, Bufomix 160/5.4, or DuoResp 160/5.4. At Step 5, LAMA is added (long-acting muscarinic antagonist) such as Spiriva Respimat with referral for assessment of phenotype. A trial of high-dose ICS-formoterol may be used. Biologic therapies may be considered such as Anti-IgE (Xolair injection), Anti-IL5/5R, Anti-IL4Ra, and Anti-TSLP.
In Track 2, the reliever inhaler is as-needed SABA, for example Ventolin, or low-dose ICS-SABA. There are currently no ICS-SABA inhalers licensed for use in Ireland.
Step 1 uses a reliever inhaler only without a maintenance inhaler. If this is a SABA like Ventolin, a low-dose ICS should be taken with each dose. Step 2 introduces a daily low-dose ICS with a reliever inhaler, but since we do not have any ICS-SABA inhalers in Ireland at present, it is very important to check that adherence is strong with the low-dose maintenance ICS inhaler. Step 3 introduces a low-dose maintenance ICS-LABA, such as Symbicort 100/6, Seretide 50 Evohaler, or Seretide 100 Diskus. Step 4 introduces a medium-dose maintenance , such as Symbicort 200/6, Seretide 125 Evohaler, or Seretide 250 Diskus. Step 5 adds on a LAMA such as Spiriva Respimat, referral for phenotype assessment and consideration of biologic anti-IG agents as per Track 1.
Track 1 with ICS-formoterol anti-inflammatory reliever inhaler is preferred because:
- It significantly reduces the risk of serious exacerbations, courses of oral corticosteroids, and the need for urgent healthcare compared to SABA-based regimes; and
- It uses a single inhaler across Steps 1-4, so it is easy to follow and easy to manage.
The table below helps identify at what Step on each Track a patient should start, depending on symptoms.
Image 2: What Step on each Track should a patient start
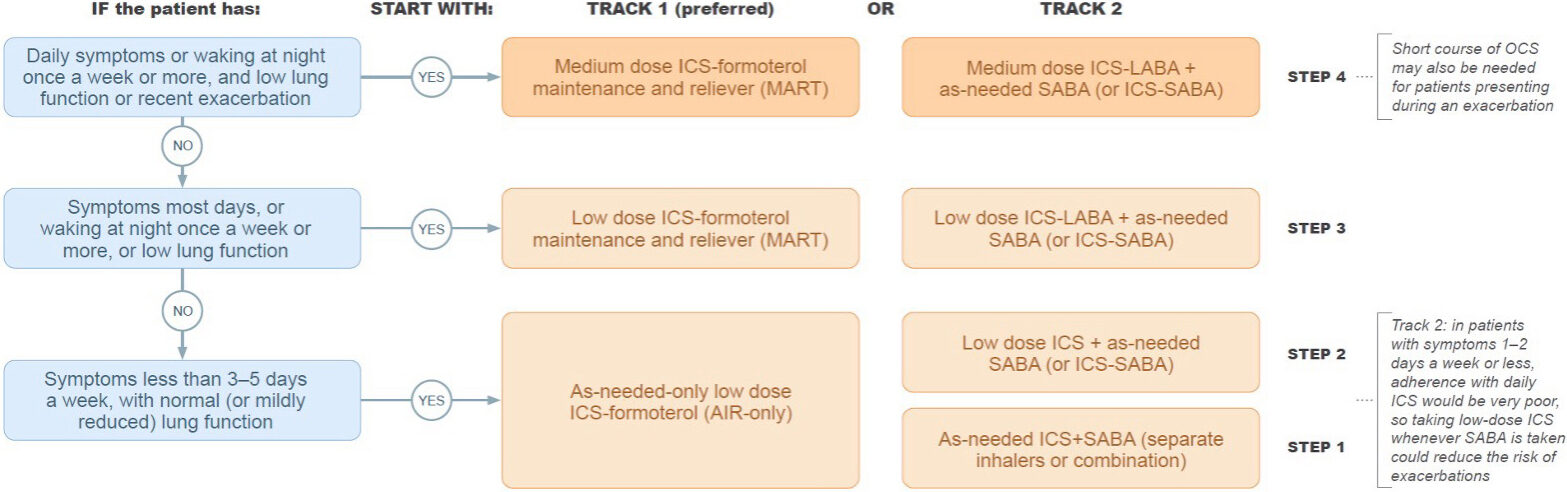 Image 3: GINA 2025 – Personalised Asthma Management
Image 3: GINA 2025 – Personalised Asthma Management
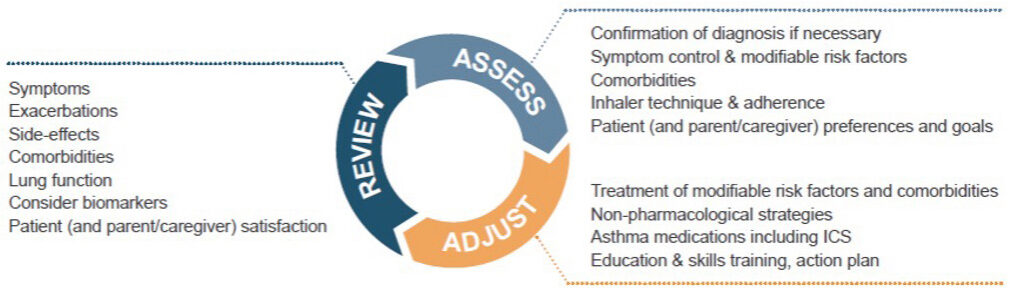
The GINA 2025 update elevated personalised asthma management to a structured, actionable strategy, tailoring every aspect of care to the individual’s biology, lifestyle, environment, and goals. The Assess-Adjust-Review cycle is a dynamic patient-centered loop that ensures treatment is effective and responsive.
At the assessment stage, a comprehensive picture is gathered of the patient’s status, including confirmation of diagnosis, symptom control, comorbidities, inhaler technique and adherence, and patient (or caregiver) preferences and goals.
At the adjustment stage, treatment is tailored based on the initial assessment. Modifiable risk factors are addressed. These include incorrect inhaler technique, poor adherence, smoking/vaping, allergen exposure, exposure to air pollutants, and obesity.
Comorbidities are also addressed and include allergic rhinitis, reflux disease, anxiety or depression, obstructive sleep apnea, and chronic sinusitis.
Asthma medications may be adjusted up or down depending on control. GINA offers two main treatment tracks, and a patient may be switched as needed. Patient needs and preferences should be considered, including their ability to correctly use a device after training, as well as availability and affordability of the device.
The review phase is a follow-up phase to explore control, adherence, and technique, to monitor side effects, and update the asthma action plan. It is a chance to review modifiable risk factors and comorbidities. The review phase can help to resolve issues early and also build trust between the patient and healthcare provider.
Conclusion
The GINA 2025 update reinforces the pharmacist’s role in delivering safe, evidence-based asthma care. By understanding the latest treatment tracks, recognising the importance of personalised management, and supporting patients through the Assess–Adjust–Review cycle, community pharmacists in Ireland can help reduce exacerbation risks and improve long-term outcomes. Staying aligned with these evolving guidelines ensures pharmacy practice remains both clinically effective and patient centered.
Further reading —’Point of Care Testing: Fractional exhaled nitric oxide’
Fractional exhaled nitric oxide (FeNO) has been proposed as a non‑invasive marker of airway inflammation in asthma. In an IPU Review article in July 2023 Lara Marín, Professional Services Pharmacist at the IPU provided an overview of the role of FeNO testing in the diagnosis and management of asthma.
Available at ipu.ie/ipu-review > Previous Articles.
Affordability is huge for people with price of some of the inhalers


 Image 3: GINA 2025 – Personalised Asthma Management
Image 3: GINA 2025 – Personalised Asthma Management

